| Pharmaceutical Information |
| Drug Name |
Adapalene |
| Drug ID |
BADD_D00042 |
| Description |
Acne vulgaris is a multifactorial disorder of the pilosebaceous unit involving increased sebum production, inflammation, and hyperproliferation/hyperkeratinization of the follicular infundibulum. It is also associated with _Cutibacterium acnes_ (also known as _Propionibacterium acnes_). Adapalene is a third-generation topical retinoid used for the treatment of acne vulgaris.[A193518] Adapalene has similar efficacy but a superior safety profile compared to tretinoin.[A193521] [Tazarotene] is more efficacious than adapalene but is designated as pregnancy category X and hence is contraindicated in pregnant women.[A193518] Adapalene can also be combined with benzoyl peroxide (BPO), which possesses bactericidal properties[A193524], and either adapalene alone, or adapalene BPO combination products, are commonly used to treat mild-to-severe acne.[A193518]
Differin®, produced by Galderma Labs, was first granted FDA approval on May 31st, 1996, as a 0.1% adapalene topical solution. Differin was later made available as 0.1% gel, cream, or lotion, or 0.3% gel products. On December 8th, 2008, Galderma Labs gained FDA approval for Epiduo®, a 0.1% adapalene, 2.5% BPO combination gel.[L12873] |
| Indications and Usage |
Adapalene is indicated for the topical treatment of acne vulgaris in patients aged 12 and over.[L12873] |
| Marketing Status |
approved |
| ATC Code |
D10AD03 |
| DrugBank ID |
DB00210
|
| KEGG ID |
D01112
|
| MeSH ID |
D000068816
|
| PubChem ID |
60164
|
| TTD Drug ID |
D0JC9N
|
| NDC Product Code |
51672-1377; 63629-8791; 0363-0888; 68308-706; 68462-403; 0299-5915; 59726-888; 66993-884; 72559-015; 72657-102; 82034-888; 15308-0716; 22365-120; 0299-5912; 0472-0126; 70000-0043; 36800-088; 0299-4910; 17337-0006; 24196-138; 53296-0068; 90027-010; 0168-0424; 49908-118; 62332-549; 46014-1098; 66039-801; 11410-956; 11673-888; 49967-354; 49967-591; 0316-0143; 71052-556; 73309-390; 45802-453; 0299-5918; 69842-088; 21130-708; 41250-288; 51672-2150 |
| UNII |
1L4806J2QF
|
| Synonyms |
Adapalene | 6-(3-(1-adamantyl)-4-methoxyphenyl)-2-naphthoic acid | CD 271 | 271, CD | CD271 | CD-271 | Adaferin | Differin | Differine |
|
| Chemical Information |
| Molecular Formula |
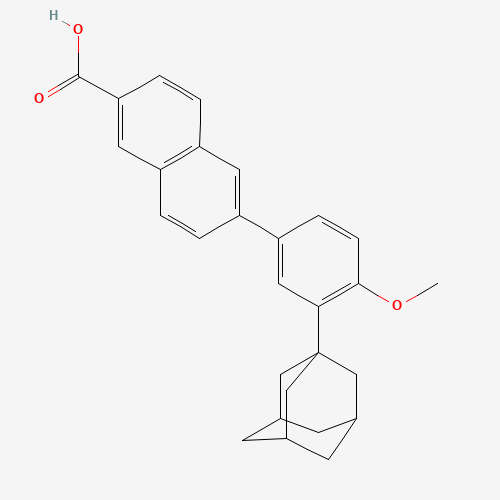
C28H28O3 |
| CAS Registry Number |
106685-40-9 |
| SMILES |
COC1=C(C=C(C=C1)C2=CC3=C(C=C2)C=C(C=C3)C(=O)O)C45CC6CC(C4)CC(C6)C5 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Skin abrasion | 23.03.11.018; 12.01.06.010 | - | - | Not Available | | Oral herpes | 11.05.02.005; 07.05.07.002 | - | - | Not Available | | Swelling of eyelid | 23.04.01.026; 10.01.05.026; 06.04.04.018 | 0.000457% | | Not Available |
|
|
|

