| Pharmaceutical Information |
| Drug Name |
Agomelatine |
| Drug ID |
BADD_D00050 |
| Description |
Agomelatine is structurally closely related to melatonin. Agomelatine is a potent agonist at melatonin receptors and an antagonist at serotonin-2C (5-HT2C) receptors, tested in an animal model of depression. Agomelatine was developed in Europe by Servier Laboratories Ltd. and submitted to the European Medicines Agency (EMA) in 2005. The Committee for Medical Products for Human Use (CHMP) recommended refusal of marketing authorization on 27 July 2006. The major concern was that efficacy had not been sufficiently shown. In 2006 Servier sold the rights to develop Agomelatine in the US to Novartis.
The development for the US market was discontinued in October 2011. It is currently sold in Australia under the Valdoxan trade name. |
| Indications and Usage |
Agomelatine is indicated to treat major depressive episodes in adults. |
| Marketing Status |
approved; investigational |
| ATC Code |
N06AX22 |
| DrugBank ID |
DB06594
|
| KEGG ID |
D02578
|
| MeSH ID |
C084711
|
| PubChem ID |
82148
|
| TTD Drug ID |
D0Y8UB
|
| NDC Product Code |
50370-0041; 50370-0053 |
| UNII |
137R1N49AD
|
| Synonyms |
agomelatine | N-(2-(7-methoxy-1-naphthyl)ethyl)acetamide | AGO 178 | AGO178 | AGO-178 | Thymanax | Valdoxan | S20098 | S 20098 | S-20098 |
|
| Chemical Information |
| Molecular Formula |
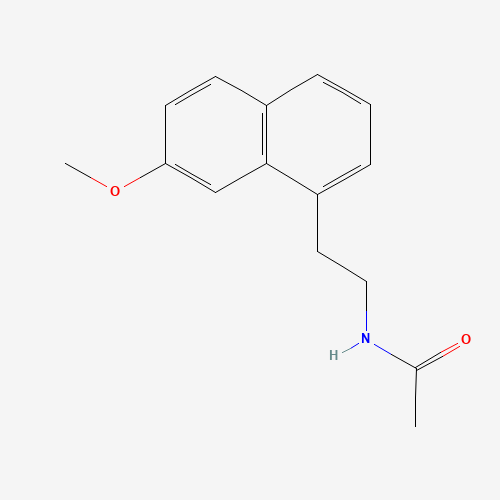
C15H17NO2 |
| CAS Registry Number |
138112-76-2 |
| SMILES |
CC(=O)NCCC1=CC=CC2=C1C=C(C=C2)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

