| Pharmaceutical Information |
| Drug Name |
Amisulpride |
| Drug ID |
BADD_D00117 |
| Description |
Amisulpride is a benzamide derivative and a dopamine receptor antagonist that selectively works on dopamine D2 and D3 receptors. As an antipsychotic agent, amisulpride alleviates both positive and negative symptoms of schizophrenia, and it exhibits antidepressant properties in patients with psychiatric disorders, dysthymia, and major depression.[A6755] Amisulpride predominantly works in the limbic system, which explains its relatively lower risk of extrapyramidal adverse effects compared to other atypical antipsychotic agents.[A6752, L32764] Oral tablets of amisulpride is used in European countries as a treatment for acute and chronic schizophrenic disorders, as well as secondary negative symptoms in mental health disorders such as affective disorders, depressive mood, and mental retardation.[L32764]
Amisulpride is also used as an antiemetic agent. In the US, the intravenous formulation of amisulpride is used to treat and prevent postoperative nausea and vomiting in adults, either as monotherapy or in combination with another antiemetic agent of a different drug class.[L32759] It is marketed under the brand name Barhemsys. |
| Indications and Usage |
Intravenous amisulpride is indicated in adults for the prevention of postoperative nausea and vomiting, either alone or in combination with an antiemetic of a different class. It is also indicated for the treatment of postoperative nausea and vomiting in patients who have received anti-emetic prophylaxis with an agent of a different class or have not received prophylaxis.[L32759]
Oral amisulpride is indicated for the treatment of acute and chronic schizophrenic disorders, characterized by positive symptoms with delusions, hallucinations, thought disorders, hostility and suspicious behavior; or primarily negative symptoms (deficit syndrome) with blunted affect, emotional and social withdrawal. Amisulpride also controls secondary negative symptoms in productive conditions as well as affective disorders such as depressive mood or retardation.[L32764] |
| Marketing Status |
approved; investigational |
| ATC Code |
N05AL05 |
| DrugBank ID |
DB06288
|
| KEGG ID |
D07310
|
| MeSH ID |
D000077582
|
| PubChem ID |
2159
|
| TTD Drug ID |
D03ELL
|
| NDC Product Code |
71390-125; 51604-1156 |
| UNII |
8110R61I4U
|
| Synonyms |
Amisulpride | 4-Amino-N-((1-ethyl-2-pyrrolidinyl)methyl)-5-(ethylsulfonyl)-2-methoxybenzamide | DAN 2163 | DAN-2163 | Solian | Sultopride | N-(Ethyl-1-pyrrolidinyl- 2-methyl)methoxy-2-ethylsulfonyl-5-benzamide | LIN 1418 | LIN-1418 | Barnetil | Sultopride Hydrochloride |
|
| Chemical Information |
| Molecular Formula |
C17H27N3O4S |
| CAS Registry Number |
71675-85-9 |
| SMILES |
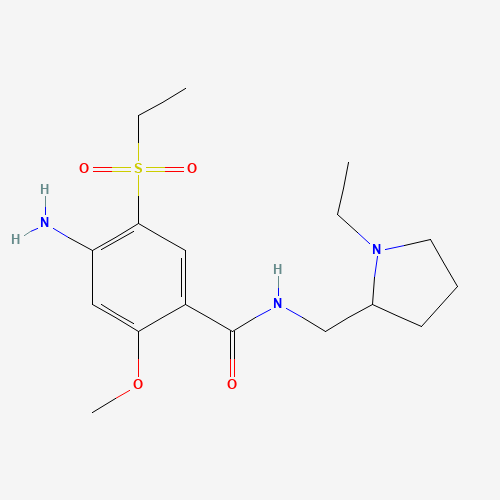
CCN1CCCC1CNC(=O)C2=CC(=C(C=C2OC)N)S(=O)(=O)CC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

