| Pharmaceutical Information |
| Drug Name |
Ammonia |
| Drug ID |
BADD_D00123 |
| Description |
Ammonia is a naturally-occurring compound with a chemical formula NH3 and structure of trigonal pyramidal geometry. It is a colorless gas with a pungent smell, and become NH4, or ammonium ion, when in water. Although ammonia is used as a food additive in the anhydrous form and serves as a starting material in pharmaceutical and commercial products, it is caustic and hazardous when concentrated. Ammonia gas has been used in the clinical setting as a respiratory stimulant to prevent fainting. The radiolabelled form of ammonia, ammonia N 13, is intravenously administered as a radioactive diagnostic agent for Positron Emission Tomography (PET) imaging of the myocardium to evaluate myocardial perfusion. Ammonia is a natural byproduct of biological and chemical reactions, including decomposition of organic matter, including plants, animals, and animal wastes. It is present in normally present in all tissues constituting a metabolic pool, where it is mostly taken up by glutamic acid and take part in transamination and other reactions, including the synthesis of protein by the Krebs-Hanseleit cycle in the liver [L2033]. It is proposed that human adults produce about 1000 mmol of ammonia daily, most of which undergoes excretion in the urine. |
| Indications and Usage |
- Indicated for use as a smelling salt to treat or prevent fainting.
- (when radiolabelled) Indicated for diagnostic PET imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in patients with suspected or existing coronary artery disease [FDA Label]. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB11118
|
| KEGG ID |
D02916
|
| MeSH ID |
D000641
|
| PubChem ID |
222
|
| TTD Drug ID |
D0FT7Y
|
| NDC Product Code |
68428-878; 55670-710 |
| UNII |
5138Q19F1X
|
| Synonyms |
Ammonia |
|
| Chemical Information |
| Molecular Formula |
H3N |
| CAS Registry Number |
7664-41-7 |
| SMILES |
N |
| Chemical Structure |
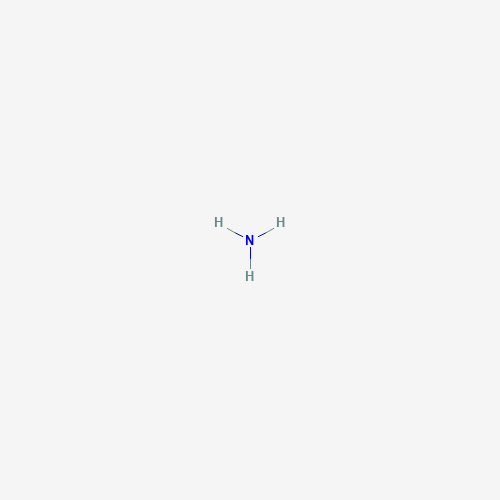
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

