| Pharmaceutical Information |
| Drug Name |
Bazedoxifene acetate |
| Drug ID |
BADD_D00215 |
| Description |
Bazedoxifene is a third generation selective estrogen receptor modulator (SERM), developed by Pfizer following the completion of their takeover of Wyeth Pharmaceuticals. In late 2013, Pfizer received approval for bazedoxifene as part of the combination drug DUAVEE in the prevention (not treatment) of postmenopausal osteoporosis. It is approved in the European Union (marketed in Italy and Spain) and Japan as monotherapy. In 2013, the combination product containing conjugated estrogens and bazedoxifene was approved by the FDA for the treatment of moderate to severe vasomotor symptoms associated with menopause, as well as the prevention of postmenopausal osteoporosis in women. |
| Indications and Usage |
Indicated for following conditions alone or in combination with conjugated estrogens in women with a uterus:
- Treatment of moderate to severe vasomotor symptoms associated with menopause
- Prevention of postmenopausal osteoporosis |
| Marketing Status |
approved; investigational |
| ATC Code |
G03XC02 |
| DrugBank ID |
DB06401
|
| KEGG ID |
D03062
|
| MeSH ID |
C447119
|
| PubChem ID |
154256
|
| TTD Drug ID |
D0JY8T
|
| NDC Product Code |
55111-949 |
| UNII |
J70472UD3D
|
| Synonyms |
bazedoxifene | TSE 424 | TSE424 | TSE-424 | WAY-140424 | bazedoxifene acetate |
|
| Chemical Information |
| Molecular Formula |
C32H38N2O5 |
| CAS Registry Number |
198481-33-3 |
| SMILES |
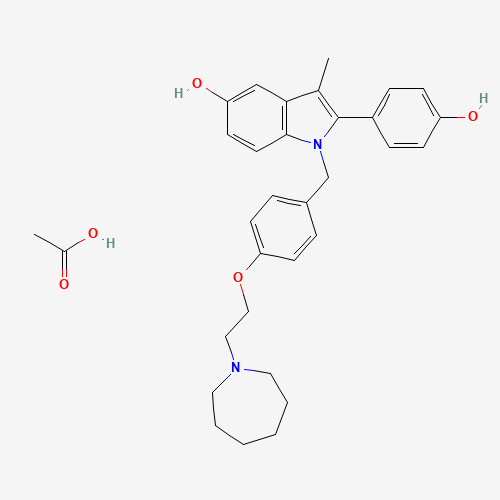
CC1=C(N(C2=C1C=C(C=C2)O)CC3=CC=C(C=C3)OCCN4CCCCCC4)C5=CC=C(C=C5)O.CC(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

