| Pharmaceutical Information |
| Drug Name |
Dothiepin hydrochloride |
| Drug ID |
BADD_D00714 |
| Description |
Dosulepin (INN, BAN) formerly known as dothiepin (USAN), is a tricyclic antidepressant with anxiolytic properties that is used in several European and South Asian countries, as well as Australia, South Africa, and New Zealand. It is not FDA-approved due to low therpeutic index and significant toxicity in overdose. Dosulepin inhibits the reuptake of biogenic amines, increasing available neurotransmitter levels at the synaptic cleft. The use of dosulepsin is only recommended in patients who are intolerant or unresponsive to alternative antidepressant therapies. Dosulepsin is a thio derivative of [DB00321] with a similar efficacy to that of [DB00321], and also exhibits anticholinergic, antihistamine and central sedative properties [L882].
Its hydrochloride form is a common active ingredient in different drug formulations. |
| Indications and Usage |
Indicated in the treatment of symptoms of depressive illness, especially where an anti-anxiety effect is required. |
| Marketing Status |
approved |
| ATC Code |
N06AA16 |
| DrugBank ID |
DB09167
|
| KEGG ID |
D01546
|
| MeSH ID |
D004308
|
| PubChem ID |
6420022
|
| TTD Drug ID |
D0Q2MN
|
| NDC Product Code |
71554-005 |
| UNII |
3H0042311V
|
| Synonyms |
Dothiepin | Dosulepin | Prothiaden | Dothiepin Hydrochloride | Hydrochloride, Dothiepin |
|
| Chemical Information |
| Molecular Formula |
C19H22ClNS |
| CAS Registry Number |
25627-39-8 |
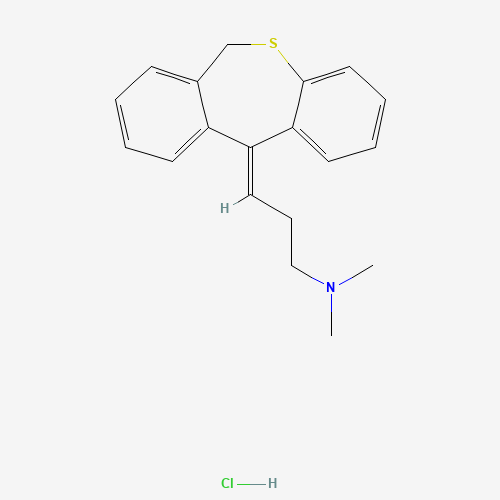
| SMILES |
CN(C)CCC=C1C2=CC=CC=C2CSC3=CC=CC=C31.Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

