| Pharmaceutical Information |
| Drug Name |
Epinastine hydrochloride |
| Drug ID |
BADD_D00781 |
| Description |
Epinastine is used for the prevention of itching associated with allergic conjunctivitis. It has a multi-action effect that inhibits the allergic response in 3 ways: 1. stabilizes mast cells by preventing mast cell degranulation to control the allergic response, 2. prevents histamine binding to both the H1- and H2-receptors to stop itching and provide lasting protection, and 3. prevents the release of proinflammatory chemical mediators from the blood vessel to halt progression of the allergic response. |
| Indications and Usage |
For the prevention of itching associated with allergic conjunctivitis. |
| Marketing Status |
approved; investigational |
| ATC Code |
R06AX24; S01GX10 |
| DrugBank ID |
DB00751
|
| KEGG ID |
D01713
|
| MeSH ID |
C053090
|
| PubChem ID |
157313
|
| TTD Drug ID |
D0DV3O
|
| NDC Product Code |
46014-1120; 33656-0017; 33656-0016; 29902-0002; 70069-008; 51991-836 |
| UNII |
GFM415S5XL
|
| Synonyms |
epinastine | 3-amino-9,13b-dihydro-1H-benz(c,f)imidazo(1,5a)azepine | Flurinol | WAL-80 Cl | WAL 801 | WAL 801 CL | WAL 801CL | epinastine hydrochloride | WAL 80 |
|
| Chemical Information |
| Molecular Formula |
C16H16ClN3 |
| CAS Registry Number |
108929-04-0 |
| SMILES |
C1C2C3=CC=CC=C3CC4=CC=CC=C4N2C(=N1)N.Cl |
| Chemical Structure |
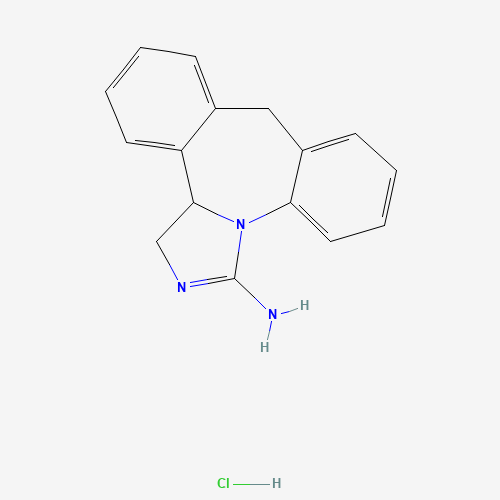
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

