| Pharmaceutical Information |
| Drug Name |
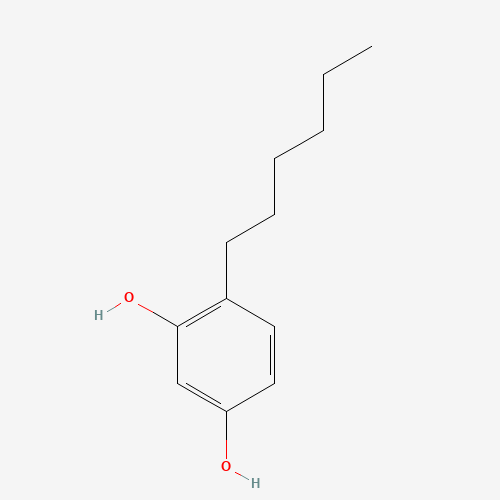
Hexylresorcinol |
| Drug ID |
BADD_D01070 |
| Description |
Hexylresorcinol is a substituted dihydroxybenzene. It exhibits antiseptic, anthelmintic, and local anesthetic properties. It can be found in topical applications for minor skin infections and in oral solutions or throat lozenges for pain relief and first aid antiseptic.
The compound may also be used commonly in various commercial cosmetic anti-aging creams while ongoing studies research the possibility of using hexylresorcinol as an anti-cancer therapy - indications all of which require further study and testing at the current moment. |
| Indications and Usage |
Hexylresorcinol is predominantly employed as the active ingredient in lotions, sprays, or lozenges indicated as a (a) topical antiseptic to help prevent skin infection in minor cuts, scrapes, or burns, or (b) as an antiseptic and local anesthetic for the relief of a sore throat and its associated pain [L2734, L2735].
In addition, hexylresorcinol is used as an active ingredient in various commercial cosmetic skincare products as an anti-aging cream [L2736] while other studies have looked into whether or not the compound could be used effectively as an anti-inflammatory agent or even as an anti-cancer therapy [L2736]. |
| Marketing Status |
approved |
| ATC Code |
R02AA12 |
| DrugBank ID |
DB11254
|
| KEGG ID |
D04441
|
| MeSH ID |
D006604
|
| PubChem ID |
3610
|
| TTD Drug ID |
D07EGB
|
| NDC Product Code |
72854-262; 72854-261; 63934-014; 69846-860 |
| UNII |
R9QTB5E82N
|
| Synonyms |
Hexylresorcinol | 4-Hexylresorcinol | 4 Hexylresorcinol |
|
| Chemical Information |
| Molecular Formula |
C12H18O2 |
| CAS Registry Number |
136-77-6 |
| SMILES |
CCCCCCC1=C(C=C(C=C1)O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Erythema | 23.03.06.001 | - | - | Not Available | | Pain | 08.01.08.004 | - | - | | | Skin irritation | 23.03.04.009 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

