| Pharmaceutical Information |
| Drug Name |
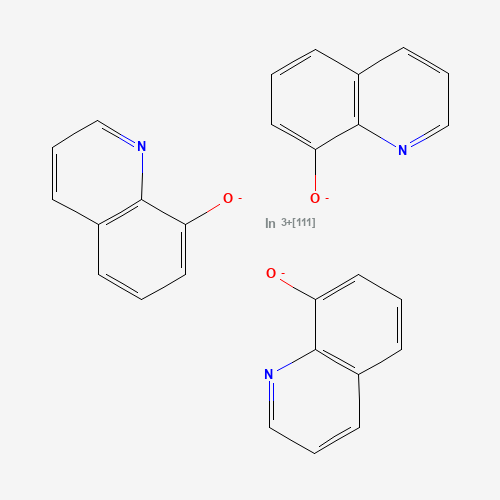
Indium in-111 oxyquinoline |
| Drug ID |
BADD_D01152 |
| Description |
Indium In 111 oxyquinoline (oxine) is a diagnostic radiopharmaceutical intended for radiolabeling of autologous leukocytes. It is composed of a 3:1 saturated complex of In-111 isotope and oxyquinoline. Indium-111 decays by isomeric transition and electron capture to cadmium-111, emitting a gamma ray that can be detected with a gamma ray camera. It is therefore useful in nuclear medicine, and is used in the labeling of leukocytes for localization of processes to which leukocytes migrate, such as those associated with abscesses or other infections. The degree of accuracy may vary with labeling techniques and with the size, location and nature of the inflammatory process.
Following intravenous administration, the lipid-soluble complex is able to penetrate platelet cell membranes. Once inside, Indium detaches from the oxyquinoline complexes and becomes attached to cytoplasmic components. |
| Indications and Usage |
Indium In 111 oxyquinoline is indicated for radiolabeling autologous leukocytes. |
| Marketing Status |
approved |
| ATC Code |
G01AC30; R02AA14; D08AH03; A01AB07 |
| DrugBank ID |
DB09473
|
| KEGG ID |
D05321; D02414
|
| MeSH ID |
D015125
|
| PubChem ID |
119117
|
| TTD Drug ID |
D02JYY
|
| NDC Product Code |
17156-021; 72536-0920 |
| UNII |
LGX9OL562T
|
| Synonyms |
Oxyquinoline | Oxyquinol | 8-Quinolinol | 8 Quinolinol | Oxine | 8-Hydroxyquinoline | 8 Hydroxyquinoline | 8-Oxyquinoline | 8 Oxyquinoline | Bioquin | Chinosol | Quinosol | Leioderm | Oxyquinoline Potassium Sulfate (2:1) | Oxyquinoline Sulfate | Sulfate, Oxyquinoline | 8-Hydroxyquinoline Sulfate | 8 Hydroxyquinoline Sulfate | Sulfate, 8-Hydroxyquinoline | Superol | Khinozol |
|
| Chemical Information |
| Molecular Formula |
C27H18InN3O3 |
| CAS Registry Number |
65389-08-4 |
| SMILES |
C1=CC2=C(C(=C1)[O-])N=CC=C2.C1=CC2=C(C(=C1)[O-])N=CC=C2.C1=CC2=C(C(=C1)[O-])N=CC
=C2.[In+3] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Pyrexia | 08.05.02.003 | - | - | | | Urticaria | 23.04.02.001; 10.01.06.001 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

