| Pharmaceutical Information |
| Drug Name |
Insulin lispro |
| Drug ID |
BADD_D01165 |
| Description |
Insulin lispro is a rapid-acting form of insulin used for the treatment of hyperglycemia caused by Type 1 and Type 2 Diabetes. Insulin is prescribed for the management of diabetes mellitus to mimic the activity of endogenously produced human insulin, a peptide hormone produced by beta cells of the pancreas that promotes glucose metabolism. Insulin is released from the pancreas following a meal to promote the uptake of glucose from the blood into internal organs and tissues such as the liver, fat cells, and skeletal muscle. Absorption of glucose into cells allows for its transformation into glycogen or fat for storage. Insulin also inhibits hepatic glucose production, enhances protein synthesis, and inhibits lipolysis and proteolysis among many other functions. |
| Indications and Usage |
Insulin lispro is indicated to improve glycemic control in adults and children with diabetes mellitus. |
| Marketing Status |
approved |
| ATC Code |
A10AB04; A10AC04; A10AD04 |
| DrugBank ID |
DB00046
|
| KEGG ID |
D04477
|
| MeSH ID |
D061268
|
| PubChem ID |
16132438
|
| TTD Drug ID |
D0L6JE
|
| NDC Product Code |
0002-0535; 0002-1455; 0002-6007; 0002-7714; 0002-0120; 0002-0121; 0002-8797; 0002-2499; 0002-7511; 0002-8222; 0002-8233; 0002-7712; 0002-8798; 0002-8799; 0024-5926; 0002-7512; 0002-7752; 0002-0124; 0002-0137; 62381-7393; 62381-9516; 50090-1375; 50090-1663; 0002-7516; 62381-7516; 0110-0541; 0024-5924; 0024-5925; 0002-7737; 0110-0571; 62381-7662; 0002-8213; 0002-1099; 0002-2576; 62381-7394; 0002-7510; 50090-5391 |
| UNII |
GFX7QIS1II
|
| Synonyms |
Insulin Lispro | Lispro, Insulin | 28(B)-Lys-29(B)-Pro-Insulin | 28(B)-Lysine-29(B)-Prolineinsulin | Insulin, Lysyl(28B)-Prolyl(28B)- | Lispro | LYSPRO | Insulin, Lys(28B)-Pro(29B)- | Humalog Kwikpen | Kwikpen, Humalog | Humalog |
|
| Chemical Information |
| Molecular Formula |
C257H389N65O77S6 |
| CAS Registry Number |
133107-64-9 |
| SMILES |
CCC(C)C(C(=O)NC(C(C)C)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)N)C(=O)NC(CS)C(=O)NC(CS)C(
=O)NC(C(C)O)C(=O)NC(CO)C(=O)NC(C(C)CC)C(=O)NC(CS)C(=O)NC(CO)C(=O)NC(CC(C)C)C(=O)
NC(CC1=CC=C(C=C1)O)C(=O)NC(CCC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(CCC(=O)O)C(=O)NC(CC(=
O)N)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(CS)C(=O)NC(CC(=O)N)C(=O)O)NC(=O)CN.CC(C)CC(C
(=O)NC(CC1=CC=C(C=C1)O)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(CS)C(=O)NCC(=O)NC(CC
C(=O)O)C(=O)NC(CCCNC(=N)N)C(=O)NCC(=O)NC(CC2=CC=CC=C2)C(=O)NC(CC3=CC=CC=C3)C(=O)
NC(CC4=CC=C(C=C4)O)C(=O)NC(C(C)O)C(=O)NC(CCCCN)C(=O)N5CCCC5C(=O)NC(C(C)O)C(=O)O)
NC(=O)C(C)NC(=O)C(CCC(=O)O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC6=CN=CN6)NC(=O
)C(CO)NC(=O)CNC(=O)C(CS)NC(=O)C(CC(C)C)NC(=O)C(CC7=CN=CN7)NC(=O)C(CCC(=O)N)NC(=O
)C(CC(=O)N)NC(=O)C(C(C)C)NC(=O)C(CC8=CC=CC=C8)N |
| Chemical Structure |
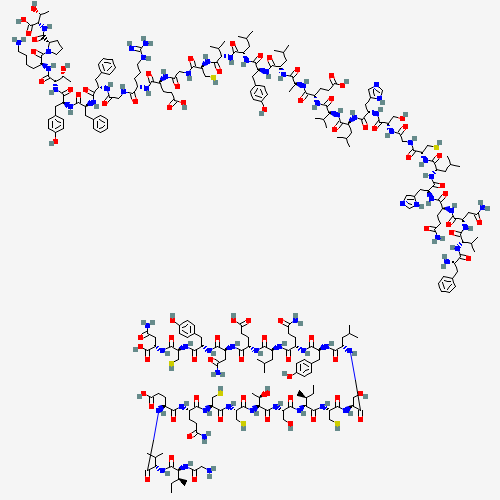
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Lipohypertrophy | 23.07.01.005; 14.08.04.009 | - | - | |
|
|
|

