| Pharmaceutical Information |
| Drug Name |
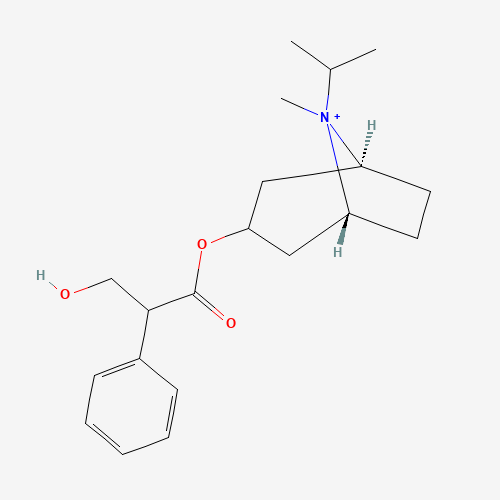
Ipratropium |
| Drug ID |
BADD_D01191 |
| Description |
Ipratropium is a quaternary ammonium derivative of [atropine][A176957] that acts as an anticholinergic agent.[A176939] It is commonly administered through inhalation which allows producing a local effect without presenting a significant systemic absorption.[A176957]
Ipratropium as a therapeutic agent was developed by Boehringer Ingelheim and its first monotherapy product was FDA approved in 1986, while the combination product of ipratropium and [albuterol] was approved in 1996.[L5894, L5891] |
| Indications and Usage |
Inhaled ipratropium is indicated in combination with inhaled beta-agonist systemic corticosteroids for the management of severe exacerbations of asthma flares requiring treatment.[A176939]
Asthma exacerbations are characterized by a progressive increase in one or more of asthma symptoms accompanied by a decrease in expiratory flow.[L5900]
As a single agent, ipratropium was indicated for the symptomatic relief of rhinorrhea associated with the common cold or seasonal allergic rhinitis for patients 5 years or older. It does not alleviate nasal congestion nor sneezing.[FDA label]
Rhinorrhea refers to recurrent or chronic watery nasal discharge. This condition is debilitating and its pathogenesis and etiology is complex and not very well understood presenting very substantial cost burden.[L5903]
Additionally, ipratropium is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease including chronic bronchitis and emphysema.[FDA label]
The chronic obstructive pulmonary disease includes a large number of conditions characterized by breathlessness. As this includes several conditions, the etiology, symptoms, and treatments are diverse.[L5906]
Ipratropium has also been studied to be used for the treatment of sialorrhea.[A176942]
Sialorrhea is a common symptom that accompanies different neurologic conditions and it is characterized by drooling or excessive salivation.[A176963] |
| Marketing Status |
approved; experimental |
| ATC Code |
R03BB01; R01AX03 |
| DrugBank ID |
DB00332
|
| KEGG ID |
D02212
|
| MeSH ID |
D009241
|
| PubChem ID |
657309
|
| TTD Drug ID |
D0S0AS
|
| NDC Product Code |
Not Available |
| UNII |
GR88G0I6UL
|
| Synonyms |
Ipratropium | (endo,syn)-(+-)-3-(3-Hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-8-azoniabicyclo(3.2.1)octane | N-Isopropylatropine | N Isopropylatropine | Atrovent | Ipratropium Bromide Anhydrous | Ipratropium Bromide, (endo,anti)-Isomer | Sch-1178 | Sch 1178 | Sch1178 | Ipratropium Bromide Monohydrate | Ipratropium Bromide, endo-Isomer | Itrop | Sch-1000 | Sch 1000 | Sch1000 | Ipratropium Bromide | Ipratropium Bromide, (exo,syn)-Isomer |
|
| Chemical Information |
| Molecular Formula |
C20H30NO3+ |
| CAS Registry Number |
60205-81-4 |
| SMILES |
CC(C)[N+]1(C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

