| Pharmaceutical Information |
| Drug Name |
Isosulfan blue |
| Drug ID |
BADD_D01212 |
| Description |
Not Available |
| Indications and Usage |
Isosulfan Blue is a synthetic visual lymphatic imaging agent. Isosulfan blue upon subcutaneous administration, delineates the lymphatic vessels draining the region of injection. It is an adjunct to lymphography in: primary and secondary lymphedema of the extremities; chyluria, chylous ascites or chylothorax; lymph node involvement by primary or secondary neoplasm; lymph node response to therapeutic modalities. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09136
|
| KEGG ID |
D04634
|
| MeSH ID |
C025484
|
| PubChem ID |
50108
|
| TTD Drug ID |
D0P2YD
|
| NDC Product Code |
14096-101; 59981-045; 69575-4004; 67457-220; 14501-0103; 68022-7525; 55150-240; 71288-805; 57821-003; 65862-911; 70069-221 |
| UNII |
39N9K8S2A4
|
| Synonyms |
iso-sulfan blue | isosulfan blue | 4-(alpha-(p-(diethylamino)phenyl)-(2,5-disulfobenzylidene)-2,5-cyclohexadien-1-ylidene)diethylammonium hydroxide inner salt, sodium salt | iso-sulphan blue | Lymphazurin | lymphazurin blue |
|
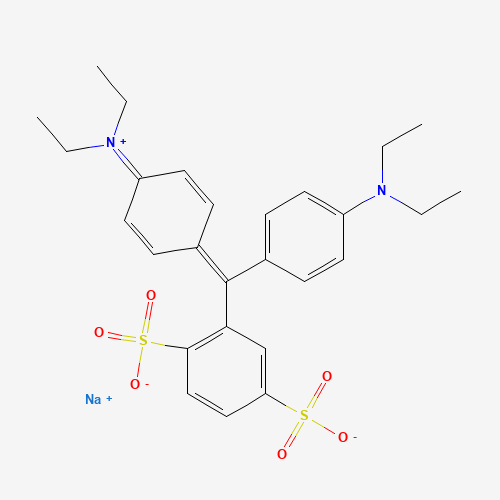
| Chemical Information |
| Molecular Formula |
C27H31N2NaO6S2 |
| CAS Registry Number |
68238-36-8 |
| SMILES |
CCN(CC)C1=CC=C(C=C1)C(=C2C=CC(=[N+](CC)CC)C=C2)C3=C(C=CC(=C3)S(=O)(=O)[O-])S(=O)
(=O)[O-].[Na+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Pruritus | 23.03.12.001 | - | - | | | Swelling | 08.01.03.015 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

