| Pharmaceutical Information |
| Drug Name |
Ivabradine hydrochloride |
| Drug ID |
BADD_D01219 |
| Description |
Ivabradine is a novel heart rate lowering medicine for the symptomatic management of stable angina pectoralis and symptomatic chronic heart failure. Ivabradine, brand name Corlanor, was approved by the FDA in April 2015 for the treatment of chronic heart failure in patients with an ejection fraction of ≤35%, in sinus rhythm with resting heart rate ≥70 beats per minute, who are not on beta-blockers due to contraindications or already receiving maximum beta-blocker dose. Recently a new indication was added to treat symptomatic heart failure from dilated cardiomyopathy for patients 6 months or more in age[Label]. Ivabradine acts by selectively inhibiting the "funny" channel pacemaker current (If) in the sinoatrial node in a dose-dependent fashion, resulting in a lower heart rate and thus more blood to flow to the myocardium. Although non-dihydropyridine calcium channel blockers and beta blockers also effectively lower heart rate, they exhibit adverse events due to their negative ionotropic effects. Therefore, as ivabradine is designed as a "pure" heart rate-lowering drug by selectively acting on the If channels, it may offer a more favorable side effect profile due to its lower likelihood of causing serious adverse effects. |
| Indications and Usage |
Ivabradine is indicated by the FDA to reduce the risk of hospitalization for worsening heart failure in adult patients with stable, symptomatic chronic heart failure with left ventricular ejection fraction ≤35%, who are in sinus rhythm with resting heart rate ≥70 beats per minute and either are on maximally tolerated doses of beta-blockers or have a contraindication to beta-blocker use. It is also indicated for treatment of stable symptomatic heart failure as a result of dilated cardiomyopathy for pediatric patients 6 months of age or more[FDA Label]. |
| Marketing Status |
approved |
| ATC Code |
C01EB17 |
| DrugBank ID |
DB09083
|
| KEGG ID |
D08095
|
| MeSH ID |
D000077550
|
| PubChem ID |
3045381
|
| TTD Drug ID |
D0S9QA
|
| NDC Product Code |
54752-0020; 59285-020; 63552-044; 63552-045; 75852-816; 65977-0098; 71666-003 |
| UNII |
TP19837BZK
|
| Synonyms |
Ivabradine | 7,8-Dimethoxy-3-(3-(((4,5-dimethoxybenzocyclobutan-1-yl)methyl)methylamino)propyl)-1,3,4,5-tetrahydro-2H-benzazepin-2-one | S 16257 | S-16257 | S16257 | S 16257-2 | S 16257 2 | S 162572 | S-16257-2 | S162572 | S-16260-2 | S162602 | S 16260-2 | S 16260 2 | S 162602 | Corlanor |
|
| Chemical Information |
| Molecular Formula |
C27H37ClN2O5 |
| CAS Registry Number |
148849-67-6 |
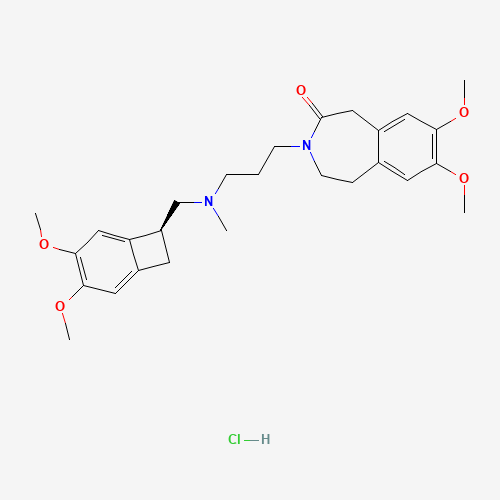
| SMILES |
CN(CCCN1CCC2=CC(=C(C=C2CC1=O)OC)OC)CC3CC4=CC(=C(C=C34)OC)OC.Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

