| Pharmaceutical Information |
| Drug Name |
Levomefolic acid |
| Drug ID |
BADD_D01278 |
| Description |
Levomefolic acid (INN) is the metabolite of folic acid (Vitamin B9) and it is a predominant active form of folate found in foods and in the blood circulation, accounting for 98% of folates in human plasma [A19276]. It is transported across the membranes including the blood-brain barrier into various tissues where it plays an essential role in the DNA synthesis, cysteine cycle and regulation of homocysteine, where it methylates homocysteine and forms methionine and tetrahydrofolate (THF). Levomefolate is approved as a food additive and is designated a GRAS (generally regarded as safe) compound [L773]. It is available commercially as a crystalline form of the calcium salt (Metafolin(R)), which has the stability required for use as a supplement [A19276]. Supplementation of levomefolic acid is desired over folic acid due to reduced potential for masking vitamin B12 deficiency symptoms. |
| Indications and Usage |
For the treatment and prevention of folate deficiency and for use as an antidote against folic acid antagonists. Contained in oral contraceptives to reduce the risk of neural tube defects arising from folic acid deficiency for pregnant women who conceived during use or shortly after the discontinuation of the product. Being studied for use as a treatment for cardiovascular diseases [A19273] and adjunct therapy for patients undergoing antidepressant pharmacotherapy [A19271, A19272]. |
| Marketing Status |
approved; investigational |
| ATC Code |
Not Available |
| DrugBank ID |
DB11256
|
| KEGG ID |
D09353
|
| MeSH ID |
C005984
|
| PubChem ID |
135398561
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
8S95DH25XC
|
| Synonyms |
5-methyltetrahydrofolate | 6S-5-methyltetrahydrofolate calcium salt | Calcium Levomefolate | mefolinate | CH3-FH4 | methyl folate | Levomefolate, calcium salt | 5-methyltetrahydropteroylglutamate | 5-methyltetrahydrofolate, (DL-Glu)-isomer | 5-methyltetrahydrofolate, calcium salt (1:1), (L-Glu)-isomer | 5-methyltetrahydrofolate, methyl-(14)C-labeled, (DL-Glu)-isomer | 5-methyltetrahydrofolate, methyl-(14)C-labeled, (L-Glu)-isomer | levomefolic acid | Prefolic A | L-methyl folate | 5-methyltetrahydrofolate, (L-Glu)-(S)-isomer | L-methylfolate | deplin | 5-methyltetrahydrofolate, (L-Glu)-(R)-isomer | N(5)-methyltetrahydrofolic acid | 5-methyltetrahydrofolic acid |
|
| Chemical Information |
| Molecular Formula |
C20H25N7O6 |
| CAS Registry Number |
31690-09-2 |
| SMILES |
CN1C(CNC2=C1C(=O)NC(=N2)N)CNC3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O |
| Chemical Structure |
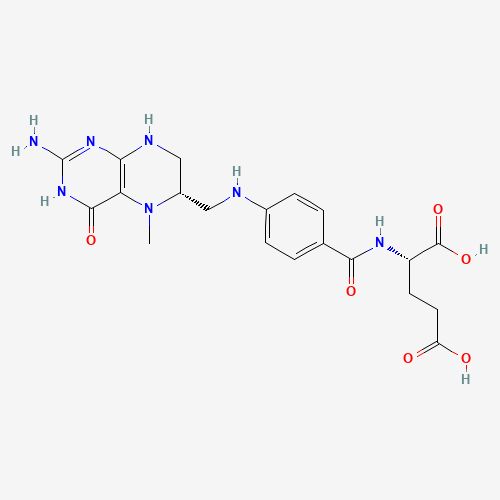
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Sensitisation | 10.02.01.012; 08.01.05.009 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

