| Pharmaceutical Information |
| Drug Name |
Meclizine |
| Drug ID |
BADD_D01361 |
| Description |
Meclizine is a histamine H1 antagonist with antiemetic and antivertigo properties. It is used in the symptomatic treatment of motion sickness and control of vertigo associated with vestibular system diseases. It also exhibits anticholinergic, central nervous system depressant, and local anesthetic effects.[L6760] Commonly marketed under the brand name Antivert in the U.S., meclizine is available as oral tablets. |
| Indications and Usage |
Indicated for the symptomatic treatment of nausea, vomiting, and dizziness associated with motion sickness,[L6772] and management of vertigo due to various causes, including radiation sickness, Meniere’s syndrome, labyrinthitis and other vestibular disturbances.[L6766] |
| Marketing Status |
approved |
| ATC Code |
R06AE05 |
| DrugBank ID |
DB00737
|
| KEGG ID |
D08163
|
| MeSH ID |
D008468
|
| PubChem ID |
4034
|
| TTD Drug ID |
D0T1XW
|
| NDC Product Code |
71554-010; 51927-0043; 50268-522; 71335-0732; 0904-6516; 65162-441; 0615-8303; 70518-1402; 67763-116 |
| UNII |
3L5TQ84570
|
| Synonyms |
Meclizine | Parachloramine | Meclozine | Antivert | Bonamine | Bonine | Chiclida | Histametizyn | Meclizine Hydrochloride | Hydrochloride, Meclizine | Meclizine Dihydrochloride | Dihydrochloride, Meclizine | Meclizine Monohydrochloride | Monohydrochloride, Meclizine | Ru-Vert-M | Ru Vert M | Agyrax | D-Vert | D Vert | DVert |
|
| Chemical Information |
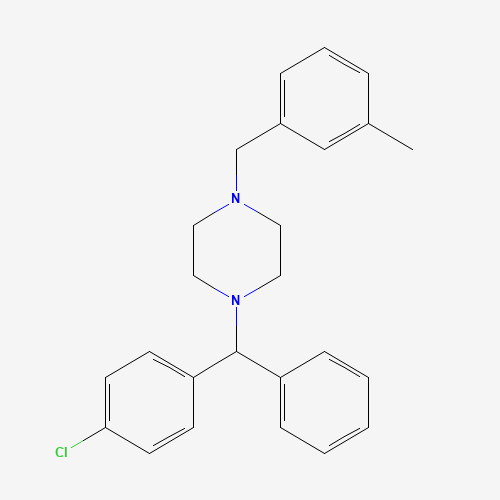
| Molecular Formula |
C25H27ClN2 |
| CAS Registry Number |
569-65-3 |
| SMILES |
CC1=CC(=CC=C1)CN2CCN(CC2)C(C3=CC=CC=C3)C4=CC=C(C=C4)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Visual acuity reduced | 17.17.01.011; 06.02.10.012 | 0.000060% | | |
|
|
|

