| Pharmaceutical Information |
| Drug Name |
Pentetate zinc trisodium |
| Drug ID |
BADD_D01723 |
| Description |
Pentetic acid, also known as diethylenetriaminepentaacetic acid (DTPA), is a synthetic polyamino carboxylic acid with eight coordinate bond forming sites that can sequester metal ions and form highly stable DTPA-metal ion complexes. DTPA, along with its calcium and zinc trisodium salts, are the only FDA approved agents for the treatment of internal contamination by transuranics.[A32501] It is currently considered, in all the dosage forms, as a member of the list of approved inactive ingredients for drug products by the FDA.[L2242] DPTA was developed by the pharmaceutical company CIS US and FDA approved on April 14, 2004.[L1469] |
| Indications and Usage |
DTPA is widely used in industry and medicine. As a medical agent, it is approved for its use in medical imaging and for the decorporation of internally deposited radionuclides.[A32501] It is FDA approved for the treatment of individuals with known or suspected internal contamination with plutonium, americium or curium to increase the rates of elimination.[L2243]
Due to the pharmacokinetic elimination by the kidneys, pentetic acid conjugated with technetium Tc-99m is being used clinically to estimate physiological parameters such as glomerular filtration rat and effective renal plasma flow.[T168] |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB14007
|
| KEGG ID |
D09762
|
| MeSH ID |
D004369
|
| PubChem ID |
164209
|
| TTD Drug ID |
D0L5QT
|
| NDC Product Code |
70651-002 |
| UNII |
NXU65IC8PG
|
| Synonyms |
Pentetic Acid | Penthanil | DTPA | Pentaind | DETAPAC | Diethylenetriamine Pentaacetic Acid | Pentaacetic Acid, Diethylenetriamine | Mn-Dtpa | Zinc-DTPA | Zinc DTPA | Ca-DTPA | CaDTPA | Pentetate Calcium Trisodium | CaNa-DTPA | Calcium Trisodium Pentetate | Pentetate, Calcium Trisodium | Pentetate Zinc Trisodium | Pentetates | Sn-DTPA | Indium-DTPA | Indium DTPA | Pentacine | Pentacin |
|
| Chemical Information |
| Molecular Formula |
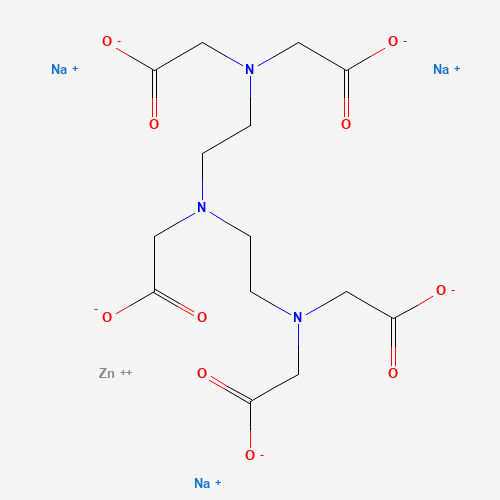
C14H18N3Na3O10Zn |
| CAS Registry Number |
11082-38-5 |
| SMILES |
C(CN(CC(=O)[O-])CC(=O)[O-])N(CCN(CC(=O)[O-])CC(=O)[O-])CC(=O)[O-].[Na+].[Na+].[N
a+].[Zn+2] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Dizziness | 02.11.04.006; 24.06.02.007; 17.02.05.003 | - | - | | | Headache | 17.14.01.001 | - | - | | | Pelvic pain | 21.10.01.001; 20.02.03.007; 07.01.06.012 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

