| Pharmaceutical Information |
| Drug Name |
Potassium citrate |
| Drug ID |
BADD_D01810 |
| Description |
Potassium citrate (also known as tripotassium citrate) is a potassium salt of citric acid. It is a white, hygroscopic crystalline powder. It is odorless with a saline taste. It contains 38.3% potassium by mass. In the monohydrate form it is highly hygroscopic and deliquescent.
Potassium citrate is used to treat a kidney stone condition called renal tubular acidosis. Potassium Citrate is indicated also for the management of Hypocitraturic calcium oxalate nephrolithiasis. |
| Indications and Usage |
For the management of renal tubular acidosis, hypocitraturic calcium oxalate nephrolithiasis, and uric acid lithiasis with or without calcium stones. |
| Marketing Status |
approved; investigational; vet_approved |
| ATC Code |
A12BA02 |
| DrugBank ID |
DB09125
|
| KEGG ID |
D05578
|
| MeSH ID |
D019357
|
| PubChem ID |
13344
|
| TTD Drug ID |
D07QPM
|
| NDC Product Code |
24196-192; 31722-129; 31722-132; 42543-407; 44523-415; 65841-538; 70518-3160; 0591-2729; 72789-203; 72789-204; 51552-0342; 42291-499; 43602-401; 51407-450; 62559-291; 68382-536; 42543-406; 63629-8759; 69452-194; 70518-1221; 0591-2682; 72789-202; 72865-192; 0178-0610; 62559-292; 63629-1966; 63629-7980; 72865-191; 0591-2742; 31722-130; 42543-408; 43602-399; 0178-0600; 63629-1967; 71335-1967; 72865-193; 0178-0615; 68084-850; 68382-537; 69452-193; 42291-500; 51407-449; 51407-451; 0245-0070; 0245-0071; 65841-536; 65841-537; 68382-538; 71335-1706; 43602-400 |
| UNII |
EE90ONI6FF
|
| Synonyms |
Potassium Citrate | Citrate, Potassium | Potassium Citrate Anhydrous | Anhydrous, Potassium Citrate |
|
| Chemical Information |
| Molecular Formula |
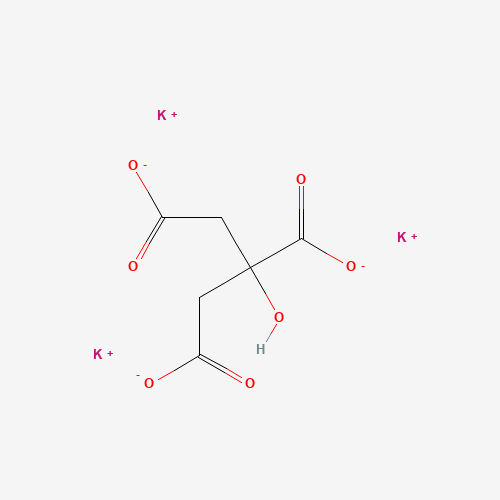
K3C6H5O7 |
| CAS Registry Number |
866-84-2 |
| SMILES |
C(C(=O)[O-])C(CC(=O)[O-])(C(=O)[O-])O.[K+].[K+].[K+] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Abdominal discomfort | 07.01.06.001 | - | - | Not Available | | Diarrhoea | 07.02.01.001 | - | - | | | Nausea | 07.01.07.001 | - | - | | | Vomiting | 07.01.07.003 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

