| Pharmaceutical Information |
| Drug Name |
Ramelteon |
| Drug ID |
BADD_D01908 |
| Description |
Ramelteon is the first in a new class of sleep agents that selectively binds to the melatonin receptors in the suprachiasmatic nucleus (SCN). It is used for insomnia, particularly delayed sleep onset. Ramelteon has not been shown to produce dependence and has shown no potential for abuse. |
| Indications and Usage |
For the treatment of insomnia characterized by difficulty with sleep onset. |
| Marketing Status |
approved; investigational |
| ATC Code |
N05CH02 |
| DrugBank ID |
DB00980
|
| KEGG ID |
D02689
|
| MeSH ID |
C495910
|
| PubChem ID |
208902
|
| TTD Drug ID |
D0U0KW
|
| NDC Product Code |
0591-2191; 59651-505; 63629-8531; 70010-028; 71205-918; 72189-369; 63415-0150; 50268-708; 42571-375; 43598-741; 52817-235; 64764-805; 72189-484; 60687-692; 70771-1495; 42413-0180; 73435-003; 50436-3980; 71335-2186; 14501-0030; 76397-021; 76397-032; 55700-874; 63629-8264; 59651-469; 66332-0014; 72189-153; 0832-1250; 42291-776; 70710-1344; 80425-0203; 80425-0343; 70700-272; 72319-005; 71335-1853; 58159-050 |
| UNII |
901AS54I69
|
| Synonyms |
ramelteon | (S)-N-(2-(1,6,7,8-tetrahydro-2H-indeno-(5,4)furan-8-yl)ethyl)propionamide | TAK-375 | Rozerem |
|
| Chemical Information |
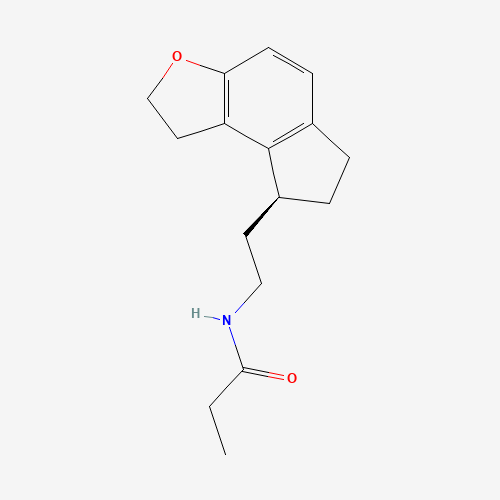
| Molecular Formula |
C16H21NO2 |
| CAS Registry Number |
196597-26-9 |
| SMILES |
CCC(=O)NCCC1CCC2=C1C3=C(C=C2)OCC3 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Cortisol decreased | 13.10.09.013 | - | - | Not Available |
|
|
|

