| Pharmaceutical Information |
| Drug Name |
Rilpivirine |
| Drug ID |
BADD_D01940 |
| Description |
Rilpivirine is non-nucleoside reverse transcriptase inhibitor (NNRTI) which is used for the treatment of HIV-1 infections in treatment-naive patients.[A31328] It is a diarylpyrimidine derivative.[A31329] The internal conformational flexibility of rilpivirine and the plasticity of it interacting binding site gives it a very high potency and reduces the chance of resistance compared to other NNRTI's.[A31331] Rilpivirine was developed by Tilbotec, Inc. and FDA approved on May 20, 2011.[L1030] On November 21, 2017, Rilpivirine, in combination with [dolutegravir], was approved as part of the first complete treatment regimen with only two drugs for the treatment of adults with HIV-1 named Juluca.[L1031] Rilpivirine in combination with [cabotegravir] was granted FDA approval on 21 January 2021.[L31193] |
| Indications and Usage |
Rilpivirine, in combination with other agents, is indicated for the treatment of HIV-1 infections in antiretroviral treatment-naive patients with HIV-1 RNA ≤100,000 copies/mL and CD4+ cell count >200 cells/mm3.[L1030] The FDA combination therapy approval of rilpivirine and dolutegravir is indicated for adults with HIV-1 infections whose virus is currently suppressed (< 50 copies/ml) on a stable regimen for at least six months, without history of treatment failure and no known substitutions associated to resistance to any of the two components of the therapy.[L1031] |
| Marketing Status |
approved |
| ATC Code |
J05AG05 |
| DrugBank ID |
DB08864
|
| KEGG ID |
D09720
|
| MeSH ID |
D000068696
|
| PubChem ID |
6451164
|
| TTD Drug ID |
D0T6WN
|
| NDC Product Code |
12578-622 |
| UNII |
FI96A8X663
|
| Synonyms |
Rilpivirine | Rilpivirine Hydrochloride | Hydrochloride, Rilpivirine | Rilpivirine HCl | HCl, Rilpivirine | R278474 | TMC 278 | 278, TMC | TMC278 | TMC-278 |
|
| Chemical Information |
| Molecular Formula |
C22H18N6 |
| CAS Registry Number |
500287-72-9 |
| SMILES |
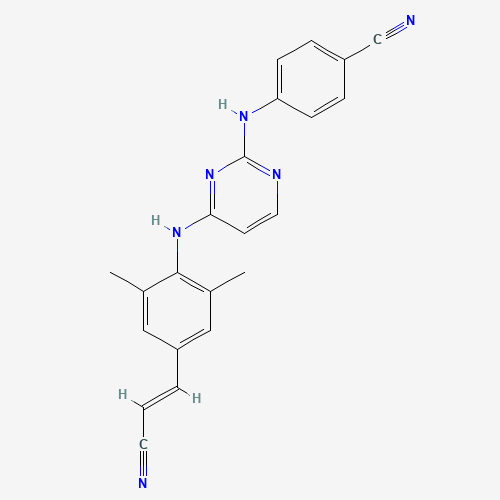
CC1=CC(=CC(=C1NC2=NC(=NC=C2)NC3=CC=C(C=C3)C#N)C)C=CC#N |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

