| Pharmaceutical Information |
| Drug Name |
Sodium carboxymethylcellulose |
| Drug ID |
BADD_D02036 |
| Description |
Carboxymethylcellulose is a cellulose derivative that consists of the cellulose backbone made up of glucopyranose monomers and their hydroxyl groups bound to carboxymethyl groups. It is added in food products as a viscosity modifier or thickener and emulsifier. It is also one of the most common viscous polymers used in artificial tears, and has shown to be effective in the treatment of aqueous tear-deficient dry eye symptoms and ocular surface staining [A33029]. The viscous and mucoadhesive properties as well as its anionic charge allow prolonged retention time in the ocular surface [A33029]. Sodium carboxymethylcellulose is the most commonly used salt. |
| Indications and Usage |
Indicated for the symptomatic relief of burning, irritation and discomfort of the eyes due to dryness or exposure to wind or sun. |
| Marketing Status |
approved; investigational |
| ATC Code |
Not Available |
| DrugBank ID |
DB11059
|
| KEGG ID |
D07622; D01544; D03608
|
| MeSH ID |
D002266
|
| PubChem ID |
6328154
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
59779-755 |
| UNII |
M28OL1HH48
|
| Synonyms |
Carboxymethylcellulose Sodium | Sodium, Carboxymethylcellulose | Carmellose Sodium | Sodium, Carmellose | Sodium Carboxymethylcellulose | Carboxymethylcellulose, Sodium | Ruspol | Aquaplast | Cellolax | Croscarmellose Sodium | Sodium, Croscarmellose | Carboxymethyl Cellulose | Cellulose, Carboxymethyl | Carboxymethylcellulose | Polycell | Aquacel | Cethylose |
|
| Chemical Information |
| Molecular Formula |
C8H16NaO8 |
| CAS Registry Number |
9004-32-4 |
| SMILES |
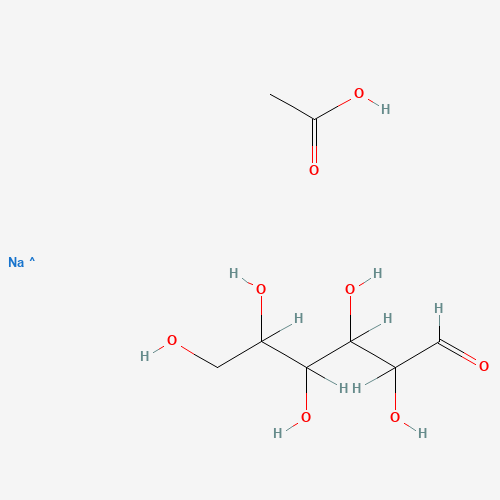
CC(=O)O.C(C(C(C(C(C=O)O)O)O)O)O.[Na] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Erythema | 23.03.06.001 | - | - | Not Available | | Eye pain | 06.08.03.002 | - | - | | | Skin irritation | 23.03.04.009 | - | - | Not Available | | Visual impairment | 06.02.10.013 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

