| Pharmaceutical Information |
| Drug Name |
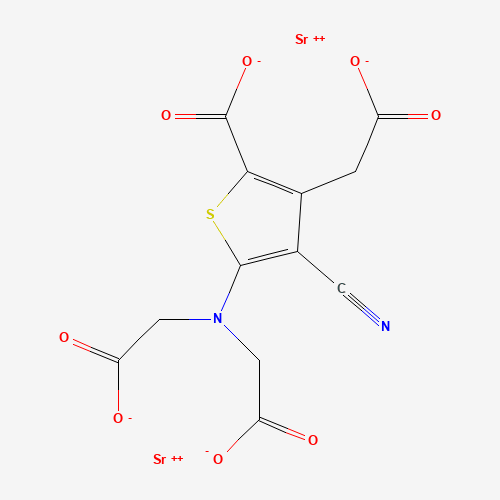
Strontium ranelate |
| Drug ID |
BADD_D02076 |
| Description |
Strontium ranelate, a strontium (II) salt of ranelic acid, is a medication for osteoporosis. Some reports have shown that strontium ranelate can slow down the progression of osteoarthritis of the knee. This agent presents an atypical mechanism of action in which it increases deposition of new bone by osteoblasts and, simultaneously, reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA) indicated for use in treatment of severe osteoporosis.
Furthermore, various clinical studies demonstrate the ability of strontium ranelate to improve and strengthen intrinsic bone tissue quality and microarchitecture in osteoporosis by way of a number of cellular and microstructural changes by which anti-fracture efficacy is enhanced.
Available for prescription use for a time in some parts of the world as Protelos (strontium ranelate) 2 g granules for oral suspension by Servier, it was ultimately discontinued in 2016-2017 owing to an increased adverse cardiac effects profile along with increased risk of venous thromboembolism (VTE) and various life threatening allergic reactions. |
| Indications and Usage |
Strontium ranelate is therapeutically indicated for the treatment of severe osteoperosis in: a) postmenopasual women, and b) adult men, who are at high risk of fractures, for whom treatment with other medical products approved for the treatment of osteoperosis is not possible due to, for example, contraindications or intolerance. [L1127]
In postmenopausal women, strontium ranelate can also reduce the risk of vertebral and hip fractures [L1127]. |
| Marketing Status |
approved; withdrawn |
| ATC Code |
M05BX03 |
| DrugBank ID |
DB09267
|
| KEGG ID |
D08468
|
| MeSH ID |
C081587
|
| PubChem ID |
6918182
|
| TTD Drug ID |
D0X7KZ
|
| NDC Product Code |
Not Available |
| UNII |
04NQ160FRU
|
| Synonyms |
strontium ranelate | protelos | S12911-5 | S12911-0 | S12911-2 | 3-(3-cyano-4-carboxymethyl-5-carboxy-2-thienyl)-3-azapentanedioic distrontium salt | S 12911 | S12911 | S-12911 |
|
| Chemical Information |
| Molecular Formula |
C12H6N2O8SSr2 |
| CAS Registry Number |
135459-87-9 |
| SMILES |
C(C1=C(SC(=C1C#N)N(CC(=O)[O-])CC(=O)[O-])C(=O)[O-])C(=O)[O-].[Sr+2].[Sr+2] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

