| Pharmaceutical Information |
| Drug Name |
Technetium tc-99m mebrofenin |
| Drug ID |
BADD_D02128 |
| Description |
Technetium Tc 99m Mebrofenin is a diagnostic radiopharmaceutical composed of diisopropyl-iminodiacetic acid (DISIDA) attached to a technetium-99m ion. Following intravenous injection, single photon emission computer tomography (SPECT) imaging of the liver or gallbladder is performed using a gamma camera to detect the gamma rays emitted by the technetium-99m as it decays. This is possible as Technetium-99m decays by isomeric transition to technetium-99 through the release of a gamma ray. Liver and gallbladder imaging is enabled through attachment to mebrofenin as this molecule has high hepatic uptake and fast biliary excretion, resulting in improved hepatic imaging. More specifically, mebrofenin is taken up into hepatocytes through the action of OATP1B1 and OATP1B3 transporters.
Currently available within a sterile kit, Tc-99m Mebrofenin is indicated for imaging of the liver and gallbladder. |
| Indications and Usage |
Technetium Tc 99m Mebrofenin is indicated as a hepatobiliary imaging agent. |
| Marketing Status |
approved |
| ATC Code |
Not Available |
| DrugBank ID |
DB09137
|
| KEGG ID |
Not Available
|
| MeSH ID |
C034837
|
| PubChem ID |
11431716
|
| TTD Drug ID |
D02AUI
|
| NDC Product Code |
Not Available |
| UNII |
F2NQ468L52
|
| Synonyms |
technetium Tc 99m mebrofenin | Tc-99m-BTM-IDA | Tc-99m-N-(2,4,6-trimethylbromoacetanilide)iminodiacetate | Tc-99m-SQ 26,962 | Tc-99m-TBIDA | Tc-99m-trimethyl-BrIDA | Tc-99m-trimethylbromo-IDA | Tc-99m-trimethylbromoimino-diacetic acid | Tc-99m-trimethylbromoiminodiacetate | Tc-TMB-IDA | technetium Tc 99m 3-bromo-2,4,6-trimethyliminodiacetic acid | technetium Tc 99m-trimethylbromo-IDA | 99mTc mebrofenin | Tc-99m-3-bromo-2,4,6-trimethyl-IDA | Tc-99m-3-bromo-2,4,6-trimethyliminodiacetic acid | Tc-99m-Br-IDA | Tc-99m-BrIDA | Choletec | Tc-Choletec | Bridatec |
|
| Chemical Information |
| Molecular Formula |
C15H19BrN2O5Tc |
| CAS Registry Number |
1415247-71-0 |
| SMILES |
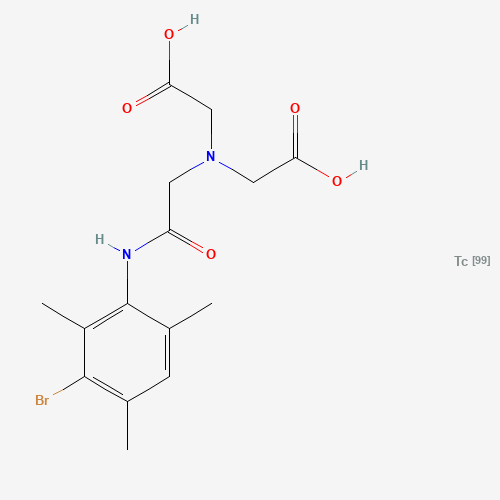
CC1=CC(=C(C(=C1NC(=O)CN(CC(=O)O)CC(=O)O)C)Br)C.[Tc] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Chills | 15.05.03.016; 08.01.09.001 | - | - | | | Death | 08.04.01.001 | - | - | | | Nausea | 07.01.07.001 | - | - | | | Rash | 23.03.13.001 | - | - | Not Available | | Urticaria | 23.04.02.001; 10.01.06.001 | - | - | |
|
The 1th Page
1
Total 1 Pages
|
|

