| Pharmaceutical Information |
| Drug Name |
Tedizolid |
| Drug ID |
BADD_D02135 |
| Description |
Drug-resistant bacteria, such as methicillin-resistant _Staphylococcus aureus_, vancomycin-resistant _Enterococcus faecium_, and penicillin-resistant _Streptococcus penumoniae_, represent a massive public health threat.[A199086, A199131] Tedizolid is a member of the oxazolidinone class of antibiotics, which includes the previously approved [linezolid] and is generally effective against multidrug-resistant Gram-positive bacteria. Tedizolid is indicated for the treatment of acute bacterial skin and skin structure infections (ABSSSI) and is generally more effective and more tolerable than [linezolid].[L11232, A199086, A199050]
Tedizolid was approved by the FDA on June 20, 2014, for sale by Cubist Pharmaceuticals as tedizolid phosphate (SIVEXTRO®). This product is currently available as both an oral tablet and as a powder for intravenous injection.[L11232] |
| Indications and Usage |
Tedizolid is indicated for the treatment of acute bacterial infections of the skin and skin structure (ABSSSI). To prevent drug resistance, tedizolid should only be used for infections that are caused by susceptible bacteria.[L11232] |
| Marketing Status |
approved; investigational |
| ATC Code |
J01XX11 |
| DrugBank ID |
DB14569
|
| KEGG ID |
D09685
|
| MeSH ID |
C546016
|
| PubChem ID |
11234049
|
| TTD Drug ID |
D08KBK
|
| NDC Product Code |
Not Available |
| UNII |
97HLQ82NGL
|
| Synonyms |
tedizolid | 3-(3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-(hydroxymethyl)oxazolidin-2-one | torezolid | 3-(3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenyl)-5-hydroxymethyloxazolidin-2-one | TR 700 | TR-700 |
|
| Chemical Information |
| Molecular Formula |
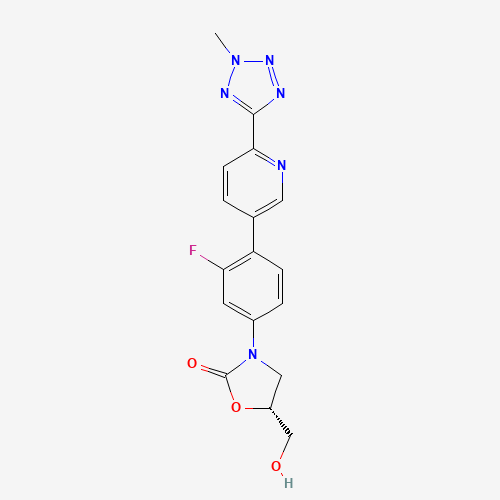
C17H15FN6O3 |
| CAS Registry Number |
856866-72-3 |
| SMILES |
CN1N=C(N=N1)C2=NC=C(C=C2)C3=C(C=C(C=C3)N4CC(OC4=O)CO)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

