| Pharmaceutical Information |
| Drug Name |
Trimethobenzamide |
| Drug ID |
BADD_D02288 |
| Description |
Trimethobenzamide is a novel antiemetic which prevents nausea and vomiting in humans. Its actions are unclear but most likely involves the chemoreceptor trigger zone (CTZ). In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate. |
| Indications and Usage |
For the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis. |
| Marketing Status |
approved; investigational |
| ATC Code |
R06AA10 |
| DrugBank ID |
DB00662
|
| KEGG ID |
D08643
|
| MeSH ID |
C100146
|
| PubChem ID |
5577
|
| TTD Drug ID |
D0A8FB
|
| NDC Product Code |
61919-884 |
| UNII |
W2X096QY97
|
| Synonyms |
trimethobenzamide | trimethobenzamide monohydrochloride | Tebamide | Ticon | Tigan | T-Gen |
|
| Chemical Information |
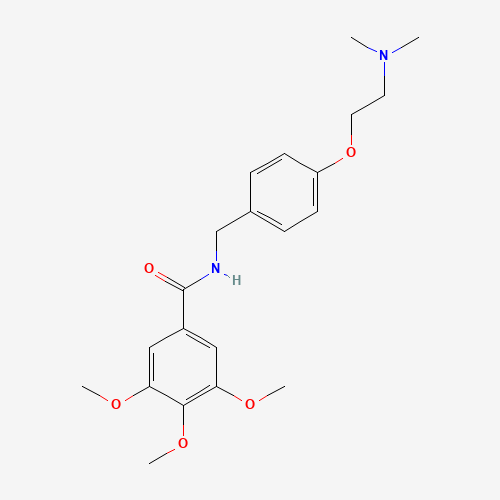
| Molecular Formula |
C21H28N2O5 |
| CAS Registry Number |
138-56-7 |
| SMILES |
CN(C)CCOC1=CC=C(C=C1)CNC(=O)C2=CC(=C(C(=C2)OC)OC)OC |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

