| Pharmaceutical Information |
| Drug Name |
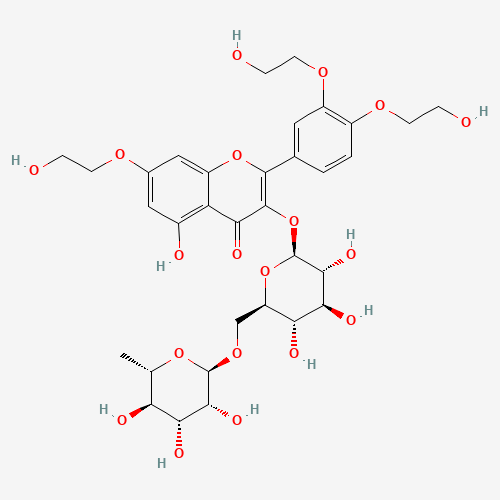
Troxerutin |
| Drug ID |
BADD_D02309 |
| Description |
Troxerutin has been used in trials studying the treatment of Chronic Venous Insufficiency. |
| Indications and Usage |
Not Available |
| Marketing Status |
investigational |
| ATC Code |
C05CA04 |
| DrugBank ID |
DB13124
|
| KEGG ID |
D07180
|
| MeSH ID |
C005865
|
| PubChem ID |
5486699
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
Not Available |
| UNII |
7Y4N11PXO8
|
| Synonyms |
troxerutin | 3',4',7-tris(O-(2- hydroxyethyl))rutin | venoruton P4 | vitamin P4 | 3',4',7-trihydroxyethylrutin | venoruton | Posorutin | Veno SL | Relvene | Rhéoflux | Teboven | Troxerutin-ratiopharm | Troxeven | Troxérutine Mazal | Vastribil | Veinamitol | Veniten retard | Venorutin | Venotrulan Trox | oxerutin | Paroven |
|
| Chemical Information |
| Molecular Formula |
C33H42O19 |
| CAS Registry Number |
7085-55-4 |
| SMILES |
CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=C(OC4=CC(=CC(=C4C3=O)O)OCCO)C5=CC(=C(C=C5)OCCO)
OCCO)O)O)O)O)O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

