| Pharmaceutical Information |
| Drug Name |
Vorapaxar sulfate |
| Drug ID |
BADD_D02368 |
| Description |
Vorapaxar is a tricyclic himbacine-derived selective inhibitor of protease activated receptor (PAR-1) indicated for reducing the incidence of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or with peripheral arterial disease (PAD). By inhibiting PAR-1, a thrombin receptor expressed on platelets, vorapaxar prevents thrombin-related platelet aggregation. |
| Indications and Usage |
Vorapaxar is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or peripheral arterial disease (PAD). It is usually co-administered with acetylsalicylic acid (ASA) and/or clopidogrel, and should therefore be administered as an addition to these medications as it has not been studied alone. |
| Marketing Status |
approved |
| ATC Code |
B01AC26 |
| DrugBank ID |
DB09030
|
| KEGG ID |
D09766
|
| MeSH ID |
C530299
|
| PubChem ID |
10077129
|
| TTD Drug ID |
D0VA0I
|
| NDC Product Code |
66992-208 |
| UNII |
IN66038E6C
|
| Synonyms |
vorapaxar | Zontivity | SCH 530348 | SCH530348 | SCH-530348 |
|
| Chemical Information |
| Molecular Formula |
C29H35FN2O8S |
| CAS Registry Number |
705260-08-8 |
| SMILES |
CCOC(=O)NC1CCC2C(C1)CC3C(C2C=CC4=NC=C(C=C4)C5=CC(=CC=C5)F)C(OC3=O)C.OS(=O)(=O)O |
| Chemical Structure |
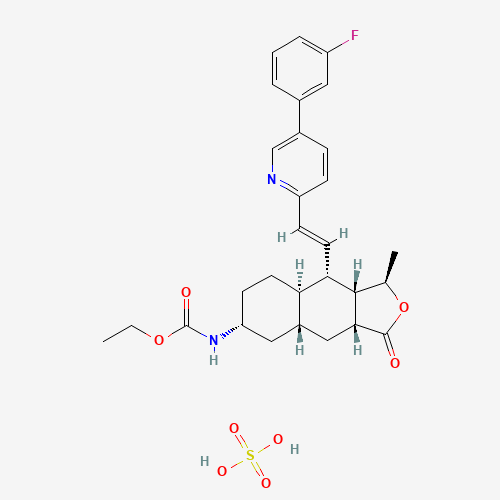
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

