| Pharmaceutical Information |
| Drug Name |
Zinc sulfate |
| Drug ID |
BADD_D02388 |
| Description |
Zinc sulfate is the inorganic compound with the formula ZnSO4 and historically known as "white vitriol". It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system. |
| Indications and Usage |
This medication is a mineral used to treat or prevent low levels of zinc alone and together with oral rehydration therapy (ORT). It is also used as a topical astringent. Zinc Sulfate Injection, USP is indicated for use as a supplement to intravenous solutions given for TPN. |
| Marketing Status |
approved; investigational |
| ATC Code |
A12CB01; B05XA18 |
| DrugBank ID |
DB09322
|
| KEGG ID |
D06371
|
| MeSH ID |
D019287
|
| PubChem ID |
24424
|
| TTD Drug ID |
D07CEI
|
| NDC Product Code |
68083-498; 68083-499; 0517-6101; 65219-403; 0517-8005; 66794-239; 65219-401; 0517-6103; 66794-240; 51927-1364; 65219-405 |
| UNII |
0J6Z13X3WO
|
| Synonyms |
Zinc Sulfate | Sulfate, Zinc | Zincteral | Zinc Sulfate, Heptahydrate |
|
| Chemical Information |
| Molecular Formula |
ZnSO4 |
| CAS Registry Number |
7733-02-0 |
| SMILES |
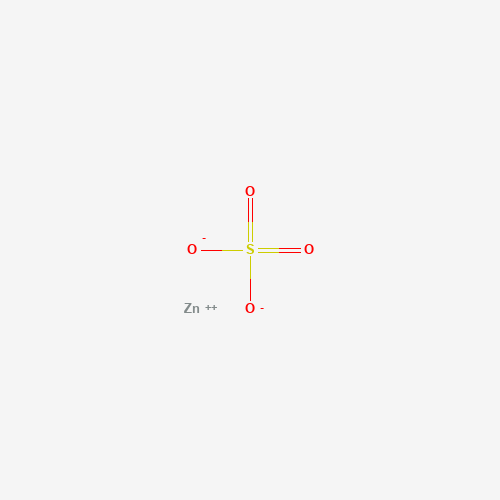
[O-]S(=O)(=O)[O-].[Zn+2] |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Nausea | 07.01.07.001 | - | - | | | Epigastric discomfort | 07.01.02.004 | - | - | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

