| Pharmaceutical Information |
| Drug Name |
Asciminib |
| Drug ID |
BADD_D02528 |
| Description |
Asciminib is a tyrosine kinase inhibitor (TKI) used in the treatment of chronic-phase Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML). More specifically, it is an inhibitor of the ABL1 kinase activity of the BCR-ABL1 fusion protein[L38995] which serves as a driver of CML proliferation in most patients with the disease.[L39005] It has also shown benefit in Ph+ CML with the T315I mutation, which produces a mutant BCR-ABL1 which is typically treatment-resistant as compared to wild-type BCR-ABL1.
Existing inhibitors of ABL compete at the ATP binding sites of these proteins and can be classified into those that target the active conformation of the kinase domain ([dasatinib], [bosutinib]) and those that target the inactive kinase domain ([imatinib], [nilotinib], [ponatinib]).[A241065] Asciminib is unique in that it acts as an allosteric inhibitor, binding at the myristoyl pocket of the BCR-ABL1 protein and locking it into an inactive conformation.[L38995,A241055]
Asciminib received FDA approval on October 29, 2021 (Scemblix, Novartis AG).[L39000] |
| Indications and Usage |
Asciminib is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase who have been previously treated with ≥2 tyrosine kinase inhibitors.[L38995] It is also indicated in the treatment of Ph+ CML in adult patients with the T315I mutation.[L38995] |
| Marketing Status |
approved; investigational |
| ATC Code |
L01EA06 |
| DrugBank ID |
DB12597
|
| KEGG ID |
D11403
|
| MeSH ID |
C000621806
|
| PubChem ID |
72165228
|
| TTD Drug ID |
DSHT18
|
| NDC Product Code |
0078-1091; 0078-1098 |
| UNII |
L1F3R18W77
|
| Synonyms |
asciminib | ABL001 | asciminib hydrochloride |
|
| Chemical Information |
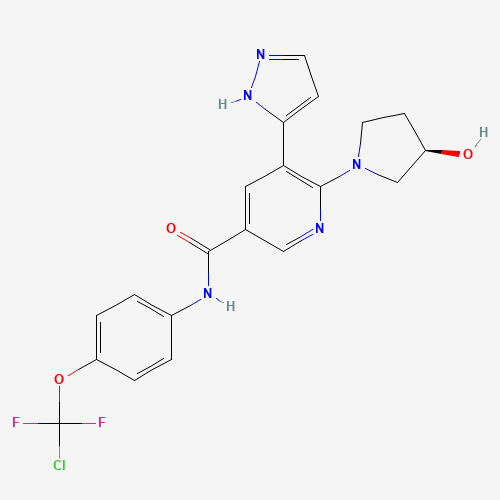
| Molecular Formula |
C20H18ClF2N5O3 |
| CAS Registry Number |
1492952-76-7 |
| SMILES |
C1CN(CC1O)C2=C(C=C(C=N2)C(=O)NC3=CC=C(C=C3)OC(F)(F)Cl)C4=CC=NN4 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

