| Pharmaceutical Information |
| Drug Name |
Atogepant |
| Drug ID |
BADD_D02529 |
| Description |
Atogepant is an oral antagonist of calcitonin gene-related peptide (CGRP) receptors indicated for the prevention of episodic migraine headaches. It was developed by AbbVie and received FDA approval under the brand name Qulipta in September 2021.[L38814] While its approval was predated by two other members of the same drug family, namely [ubrogepant] and [rimegepant], these agents are indicated only for abortive migraine therapy - atogepant is novel in that it is the first and only oral CGRP antagonist approved for preventative use in migraine.[L38814]
In patients requiring preventative migraine therapy, current practice guidelines recommend the use of certain anti-epileptic medications (e.g. [valproic acid] or [topiramate]) or beta-blockers (e.g. [propranolol]), all of which can be associated with significant adverse effects.[A239094] The "gepants" family of drugs, including atogepant, are comparatively well-tolerated[A189207,L38739] and may provide a desirable treatment option for patients struggling with adverse reactions to other preventative therapies. |
| Indications and Usage |
Atogepant is indicated for the prevention of episodic migraine in adults.[L38739] |
| Marketing Status |
approved; investigational |
| ATC Code |
N02CD07 |
| DrugBank ID |
DB16098
|
| KEGG ID |
D11313
|
| MeSH ID |
C000718987
|
| PubChem ID |
72163100
|
| TTD Drug ID |
D02ZUG
|
| NDC Product Code |
0074-7093; 0074-7092; 0074-7091; 0074-7096; 0074-7094; 0074-7095 |
| UNII |
7CRV8RR151
|
| Synonyms |
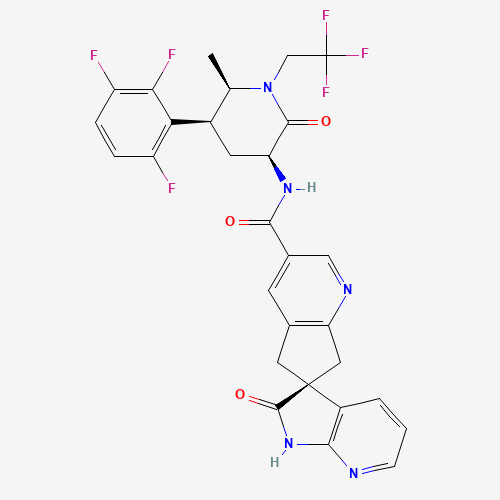
atogepant | (3S)-N-((3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl)-2-oxospiro(1H-pyrrolo(2,3-b)pyridine-3,6'-5,7-dihydrocyclopenta(b)pyridine)-3'-carboxamide |
|
| Chemical Information |
| Molecular Formula |
C29H23F6N5O3 |
| CAS Registry Number |
1374248-81-3 |
| SMILES |
CC1C(CC(C(=O)N1CC(F)(F)F)NC(=O)C2=CC3=C(CC4(C3)C5=C(NC4=O)N=CC=C5)N=C2)C6=C(C=CC
(=C6F)F)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

