| Pharmaceutical Information |
| Drug Name |
Calcifediol |
| Drug ID |
BADD_D02538 |
| Description |
The major circulating metabolite of vitamin D3 (cholecalciferol). It is produced in the liver and is the best indicator of the body's vitamin D stores. It is effective in the treatment of rickets and osteomalacia, both in azotemic and non-azotemic patients. Calcifediol also has mineralizing properties. |
| Indications and Usage |
Used to treat vitamin D deficiency or insufficiency, refractory rickets (vitamin D resistant rickets), familial hypophosphatemia and hypoparathyroidism, and in the management of hypocalcemia and renal osteodystrophy in patients with chronic renal failure undergoing dialysis. Also used in conjunction with calcium in the management and prevention of primary or corticosteroid-induced osteoporosis. |
| Marketing Status |
approved; nutraceutical |
| ATC Code |
A11CC06; H05BX05 |
| DrugBank ID |
DB00146
|
| KEGG ID |
D00122
|
| MeSH ID |
D002112
|
| PubChem ID |
5283731
|
| TTD Drug ID |
D02VPX
|
| NDC Product Code |
45408-002; 11014-0440; 66499-0065; 70301-1001; 11014-0204 |
| UNII |
P6YZ13C99Q
|
| Synonyms |
Calcifediol | 25-Hydroxyvitamin D 3 | 25 Hydroxyvitamin D 3 | 25-Hydroxycholecalciferol Monohydrate | 25 Hydroxycholecalciferol Monohydrate | Monohydrate, 25-Hydroxycholecalciferol | 25-Hydroxyvitamin D3 | 25 Hydroxyvitamin D3 | Calcidiol | 25-Hydroxycholecalciferol | 25 Hydroxycholecalciferol | Calcifediol, (3 beta,5E,7E)-Isomer | Calcifediol Anhydrous | Anhydrous, Calcifediol | Dedrogyl | Hidroferol | Calcifediol, (3 alpha,5Z,7E)-Isomer | Calderol |
|
| Chemical Information |
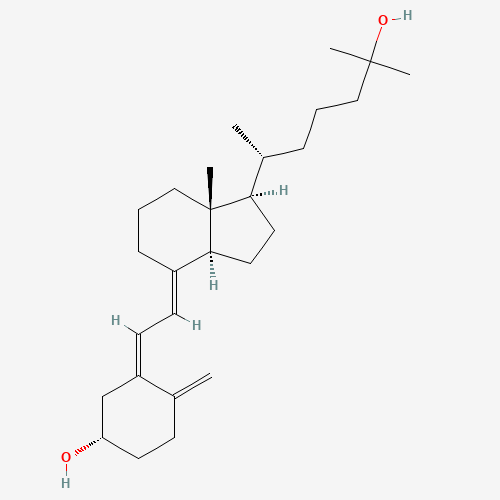
| Molecular Formula |
C27H44O2 |
| CAS Registry Number |
19356-17-3 |
| SMILES |
CC(CCCC(C)(C)O)C1CCC2C1(CCCC2=CC=C3CC(CCC3=C)O)C |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Death | 08.04.01.001 | 0.043465% | | | | Dizziness | 02.11.04.006; 24.06.02.007; 17.02.05.003 | 0.006520% | | | | Dry skin | 23.03.03.001 | 0.002415% | | | | Dyspnoea | 02.11.05.003; 22.02.01.004 | 0.002415% | | | | Erythema | 23.03.06.001 | 0.002415% | | Not Available | | Pruritus | 23.03.12.001 | 0.002415% | | | | Renal failure | 20.01.03.005 | 0.005312% | | Not Available |
|
The 1th Page
1
Total 1 Pages
|
|

