| Pharmaceutical Information |
| Drug Name |
Darolutamide |
| Drug ID |
BADD_D02548 |
| Description |
Darolutamide is a nonsteroidal androgen receptor antagonist for the treatment of castrate-resistant, non-metastatic prostate cancer (nmCRPC). This condition occurs in the majority of patients with advanced prostate cancer who have been treated with androgen receptor antagonists.[A189063] Though prior treatment for prostate cancer has been successful for these patients, the cancer eventually progresses to become resistant to existing therapies. This warrants further treatment.
The goal of treatment with darolutamide is to delay the progression of prostate cancer to metastatic disease, increasing quality of life and life expectancy for those with advanced prostate cancer.[A189054,A189063] Darolutamide was developed by Bayer HealthCare Pharmaceuticals Inc. and approved by the FDA on July 30th, 2019.[L10887] |
| Indications and Usage |
This drug is indicated for the treatment of patients diagnosed with non-metastatic and castrate-resistant prostate cancer.[L10872] |
| Marketing Status |
approved; investigational |
| ATC Code |
L02BB06 |
| DrugBank ID |
DB12941
|
| KEGG ID |
D11045
|
| MeSH ID |
C000607739
|
| PubChem ID |
67171867
|
| TTD Drug ID |
D0DV6D
|
| NDC Product Code |
54893-0107; 50419-395; 52483-6300 |
| UNII |
X05U0N2RCO
|
| Synonyms |
darolutamide | Nubeqa | ORM-16497 | ODM-201 | ORM-16555 |
|
| Chemical Information |
| Molecular Formula |
C19H19ClN6O2 |
| CAS Registry Number |
1297538-32-9 |
| SMILES |
CC(CN1C=CC(=N1)C2=CC(=C(C=C2)C#N)Cl)NC(=O)C3=NNC(=C3)C(C)O |
| Chemical Structure |
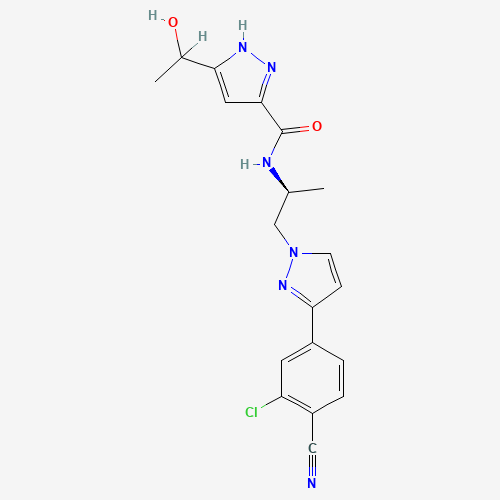
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Taste disorder | 17.02.07.029; 07.14.03.004 | 0.000734% | | Not Available |
|
|
|

