| Pharmaceutical Information |
| Drug Name |
Docusate |
| Drug ID |
BADD_D02551 |
| Description |
Docusate, or dioctyl sulfosuccinate, is a stool softener indicated for the treatment of constipation[A32201]. Docusate acts by increasing the amount of water the stool absorbs in the gut, making the stool softer and easier to pass [L1801]. Docusate can be orally or rectally administered. Docusate is on the World Health Organization's List of Essential Medicines[L5915]. However the effectiveness of docusate in treating constipation remains unclear, as several studies report docusate to be no more effective than placebo for increasing the frequency of stool or stool softening [A32200,A32202,A176987]. Recently there has been pressure to stop prescribing docusate as it has been identified as an ineffective medicine[A176972,A176987,L5912]. Additionally, it does not appear to lessen symptoms associated with constipation such as abdominal cramps. Still docusate is available in over-the-counter products as a common laxative. |
| Indications and Usage |
Indicated for the treatment of constipation associated with dry, hard stools or opioid induced constipation[A176984]. Though recently, pressure has been building to end the use of docusate over concerns of efficacy[A176972,A176987,L5912]. |
| Marketing Status |
approved |
| ATC Code |
A06AA02 |
| DrugBank ID |
DB11089
|
| KEGG ID |
D00305; D03885; D03886
|
| MeSH ID |
D004143
|
| PubChem ID |
11339
|
| TTD Drug ID |
D0X4FM
|
| NDC Product Code |
Not Available |
| UNII |
M7P27195AG
|
| Synonyms |
Dioctyl Sulfosuccinic Acid | Sulfosuccinic Acid bis(2-Ethylhexyl) Ester | Colace | Dioctyl Sulfosuccinates | Sulfosuccinates, Dioctyl | Dioctyl Sulfosuccinic Acid, Ammonium Salt | Dioctyl Sulfosuccinic Acid, Barium Salt | Docusate | Dioctylsulfosuccinate | DOSS | Dioctyl Sulfosuccinate | Sulfosuccinate, Dioctyl | Docusate Calcium | Dioctyl Sulfosuccinic Acid, Calcium Salt | Docusate Potassium | Dioctyl Sulfosuccinic Acid, Potassium Salt | Docusate Sodium | Dioctyl Sulfosuccinic Acid, Sodium Salt | Sodium Bis(2-ethylhexyl)sulfosuccinate | DEH-Na-SS | DEH Na SS | Sodium Dioctylsulphosuccinate | Dioctylsulphosuccinate, Sodium | Diethylhexyl Sodium Sulfosuccinate | Sodium Sulfosuccinate, Diethylhexyl | Sulfosuccinate, Diethylhexyl Sodium | Sodium Dioctyl Sulfosuccinate | Dioctyl Sulfosuccinate, Sodium | Aerosol OT | Dioctyl Sulfosuccinic Acid, Magnesium Salt |
|
| Chemical Information |
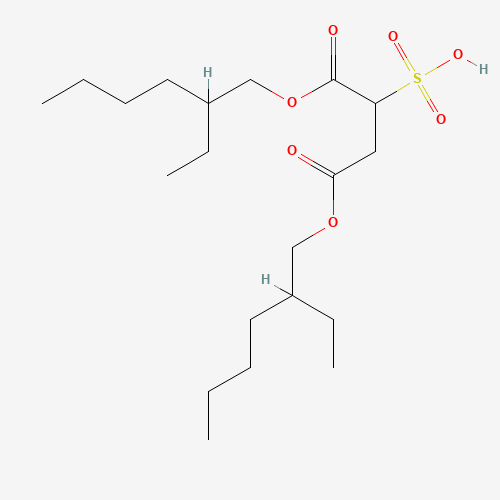
| Molecular Formula |
C20H38O7S |
| CAS Registry Number |
10041-19-7 |
| SMILES |
CCCCC(CC)COC(=O)CC(C(=O)OCC(CC)CCCC)S(=O)(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Somnolence | 19.02.05.003; 17.02.04.006 | 0.000121% | | | | Urticaria | 23.04.02.001; 10.01.06.001 | - | - | | | Vomiting | 07.01.07.003 | 0.000055% | | | | Faecaloma | 07.01.03.004 | 0.000055% | | Not Available | | Oropharyngeal pain | 22.12.03.016; 07.05.05.004 | 0.000121% | | | | Fixed eruption | 10.01.01.037; 08.01.06.025; 23.03.05.008 | - | - | Not Available |
|
|
|

