| Pharmaceutical Information |
| Drug Name |
Lasmiditan |
| Drug ID |
BADD_D02562 |
| Description |
Lasmiditan is an oral medication used in the termination of migraine headaches that was first approved for use in the United States in October 2019.[L9338,L9356]
Traditionally, the triptan class of anti-migraine medications (e.g. [sumatriptan]) have seen preferential use in the acute treatment of migraines due to their relatively favourable efficacy and safety. Their use is not devoid of concerns, however, and their vasoconstrictive activity can lead to blood pressure lability and other cardiovascular side effects - for this reason, these medications are less suitable for use in patients with pre-existing cardiovascular disorders.[A187316] Triptans abort migraines via action at several serotonin receptors, including 5-HT1D and 5-HT1B receptors, and activity at the 5-HT1B receptor has been specifically implicated in their vasoconstrictive activity.[A187316,A187322]
Lasmiditan, in contrast, is a highly selective agonist of 5-HT1F receptors, carrying virtually no affinity for other receptors which appear to be largely responsible for the adverse effect profile of its predecessors - in other words, lasmiditan’s selectivity allows for the successful termination of migraines without causing vasoconstriction.[A187322,A187319] Selectivity for 5-HT1F, a lack of vasoconstrictive activity, and the ability to terminate migraines through neuronal inhibition has resulted in the creation of a new class of anti-migraine medications in which lasmiditan is the first and only member: the neurally-acting anti-migraine medications (NAAMAs).[A187322,A187307] |
| Indications and Usage |
Lasmiditan is indicated for the acute treatment of migraine with or without aura in adults.[L9338] |
| Marketing Status |
approved; investigational |
| ATC Code |
N02CC08 |
| DrugBank ID |
DB11732
|
| KEGG ID |
D10338
|
| MeSH ID |
C554777
|
| PubChem ID |
11610526
|
| TTD Drug ID |
D01NQM
|
| NDC Product Code |
0002-4312; 0002-4491; 0110-4491; 0110-4312 |
| UNII |
760I9WM792
|
| Synonyms |
lasmiditan | 2,4,6-trifluoro-N-(6-((1-methylpiperidin-4-yl)carbonyl)pyridin-2yl)benzamide | Reyvow | lasmiditan hydrochloride | COL-144 | LY573144 |
|
| Chemical Information |
| Molecular Formula |
C19H18F3N3O2 |
| CAS Registry Number |
439239-90-4 |
| SMILES |
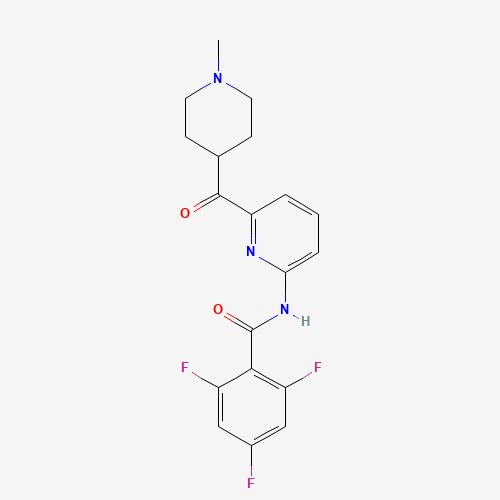
CN1CCC(CC1)C(=O)C2=NC(=CC=C2)NC(=O)C3=C(C=C(C=C3F)F)F |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

