| Pharmaceutical Information |
| Drug Name |
Pitolisant |
| Drug ID |
BADD_D02583 |
| Description |
Pitolisant is a selective antagonist or inverse agonist of the histamine H3 receptor used to treat type 1 or 2 narcolepsy.[L1471,L8063] Narcolepsy is a chronic neurological disorder that affects 1 in 2,000 individuals and is characterized by excessive daytime sleepiness, abnormal REM sleep manifestations, sleep paralysis and hypnagogic hallucinations.[A32025] About 60-70% of patients with narcolepsy experience cataplexy, which is a sudden loss of muscle tone triggered by positive or negative emotions.[A32022] Histaminergic neuron signalling in the brain plays a role in maintaining wakefulness; by blocking histamine autoreceptors, pitolisant enhances the activity of histaminergic neurons, as well as increasing the signalling of other neurotransmitters in the brain.[L1471]
In a European clinical trial of adult patients with narcolepsy, there was a reduction in the Epworth Sleepiness Scale (ESS) score from pitolisant therapy compared to placebo.[A32024] The therapeutic effectiveness of pitolisant was comparable to that of [modafinil].[A32024] Pitolisant therapy was also effective in treating refractory sleepiness in adolescent patients with narcolepsy, where it decreased ESS score and increased the mean sleep onset latency.[A32023] Adolescent patients with cataplexy also experienced a slight improvement in the frequency and severity of symptoms [A32023]; however, the safety of use in adolescent or paediatric patients have not been established with pitolisant. Commonly marketed under the trade name Wakix, oral pitolisant was approved by the EMA in 2016 [A183062] for the treatment of narcolepsy with or without cataplexy.[L1471] FDA approved the use of pitolisant in 2019 for excessive daytime sleepiness (EDS) associated with narcolepsy in adults.[L8063] |
| Indications and Usage |
Pitolisant is indicated for the treatment of narcolepsy with or without cataplexy [L1471] and excessive daytime sleepiness in narcolepsy in adult patients.[L8063] |
| Marketing Status |
approved; investigational |
| ATC Code |
N07XX11 |
| DrugBank ID |
DB11642
|
| KEGG ID |
D10749
|
| MeSH ID |
C516975
|
| PubChem ID |
9948102
|
| TTD Drug ID |
D01UUD
|
| NDC Product Code |
Not Available |
| UNII |
4BC83L4PIY
|
| Synonyms |
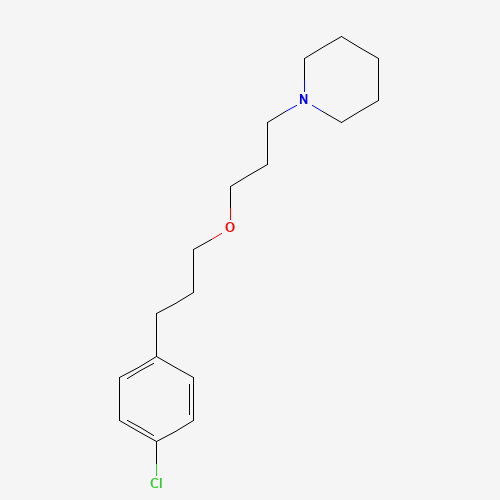
pitolisant | 1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine | BF2.649 | tiprolisant | Wakix |
|
| Chemical Information |
| Molecular Formula |
C17H26ClNO |
| CAS Registry Number |
362665-56-3 |
| SMILES |
C1CCN(CC1)CCCOCCCC2=CC=C(C=C2)Cl |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

