| Pharmaceutical Information |
| Drug Name |
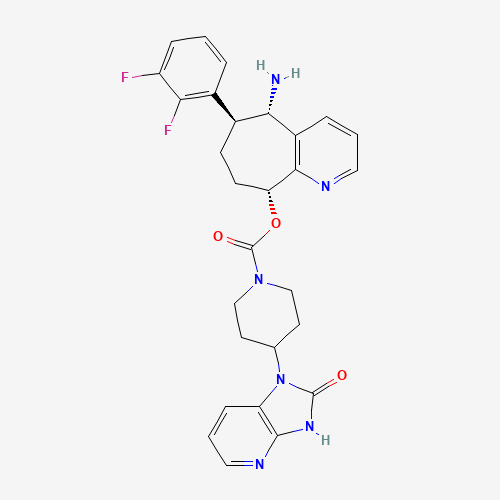
Rimegepant |
| Drug ID |
BADD_D02590 |
| Description |
Rimegepant is an oral antagonist of the CGRP receptor developed by Biohaven Pharmaceuticals.[L11028] It received FDA approval on February 27, 2020 for the acute treatment migraine headache.[L11974] While several parenteral antagonists of CGRP and its receptor have been approved for migraine therapy (e.g. [erenumab], [fremanezumab], [galcanezumab]), rimegepant and [ubrogepant] were the only CGRP antagonists that possessed oral bioavailability[A189207] until the approval of [atogepant] in 2021.[L38814]
The current standard of migraine therapy involves abortive treatment with "triptans", such as [sumatriptan], but these medications are contraindicated in patients with pre-existing cerebrovascular and cardiovascular disease due to their vasoconstrictive properties.[A189207] Antagonism of the CGRP pathway has become an attractive target for migraine therapy as, unlike the triptans, oral CGRP antagonists have no observed vasoconstrictive properties and are therefore safer for use in patients with contraindications to standard therapy.[A189330,A189207] |
| Indications and Usage |
Rimegepant is indicated for the acute treatment of migraine with or without aura in adults.[L11971] |
| Marketing Status |
approved; investigational |
| ATC Code |
N02CD06 |
| DrugBank ID |
DB12457
|
| KEGG ID |
D10662
|
| MeSH ID |
C578443
|
| PubChem ID |
51049968
|
| TTD Drug ID |
D0V9VG
|
| NDC Product Code |
46016-1370 |
| UNII |
997WVV895X
|
| Synonyms |
rimegepant sulfate | rimegepant | BMS-927711 | (5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta(b)pyridin-9-yl 4-(2-oxo-2,3-dihydro-1H-imidazo(4,5-b)pyridin-1-yl)piperidine-1-carboxylate | Nurtec ODT |
|
| Chemical Information |
| Molecular Formula |
C28H28F2N6O3 |
| CAS Registry Number |
1289023-67-1 |
| SMILES |
C1CC(C2=C(C=CC=N2)C(C1C3=C(C(=CC=C3)F)F)N)OC(=O)N4CCC(CC4)N5C6=C(NC5=O)N=CC=C6 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

