| Pharmaceutical Information |
| Drug Name |
Tucatinib |
| Drug ID |
BADD_D02603 |
| Description |
Tucatinbib is a kinase inhibitor drug used with [trastuzumab] and [capecitabine] in the treatment of unresectable or metastatic HER-2 positive breast cancer. It was developed by Seattle Genetics and approved by the FDA on April 17, 2020.[L12951] Tucatinib is a promising new treatment for patients with metastatic breast cancer who have not responded adequately to other chemotherapy regimens.[L12945] |
| Indications and Usage |
Tucatinib is indicated with trastuzumab and capecitabine for treatment of adults diagnosed with advanced unresectable or metastatic HER2-positive breast cancer. This includes patients with brain metastases and those who have received one or more prior anti-HER2-based regimens in the metastatic setting.[L12945] |
| Marketing Status |
approved; investigational |
| ATC Code |
L01EH03 |
| DrugBank ID |
DB11652
|
| KEGG ID |
D11141
|
| MeSH ID |
C000705452
|
| PubChem ID |
51039094
|
| TTD Drug ID |
D09GRX
|
| NDC Product Code |
11014-0476; 43265-7481; 43265-7480; 51144-002; 57572-0723; 51144-001 |
| UNII |
234248D0HH
|
| Synonyms |
tucatinib | irbinitinib | ONT-380 | tukysa | N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine | N6-(4,5-dihydro-4,4-dmethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-A)pyridin-7-Yloxy)phenyl)-4,6-quinazolinediamine |
|
| Chemical Information |
| Molecular Formula |
C26H24N8O2 |
| CAS Registry Number |
937263-43-9 |
| SMILES |
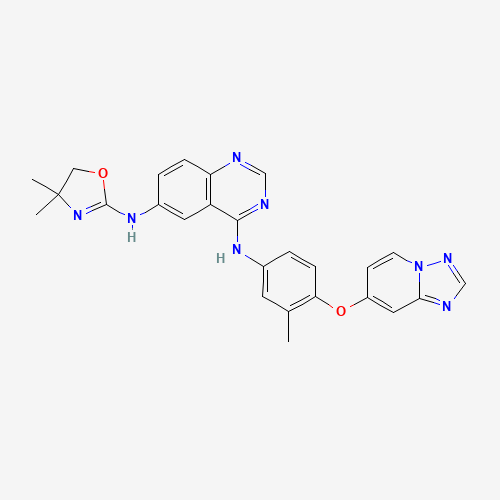
CC1=C(C=CC(=C1)NC2=NC=NC3=C2C=C(C=C3)NC4=NC(CO4)(C)C)OC5=CC6=NC=NN6C=C5 |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Taste disorder | 17.02.07.029; 07.14.03.004 | 0.000817% | | Not Available | | Treatment noncompliance | 08.06.01.067; 12.09.02.006 | 0.000246% | | Not Available |
|
|
|

