| Pharmaceutical Information |
| Drug Name |
Bilastine |
| Drug ID |
BADD_D02615 |
| Description |
Bilastine is a novel new-generation antihistamine that is highly selective for the H1 histamine receptor, has a rapid onset and prolonged duration of action. |
| Indications and Usage |
For symptomatic relief of nasal and non-nasal symptoms of seasonal rhinitis in patients 12 years of age and older and for symptomatic relief in chronic spontaneous urticaria in patients 18 years of age and older [FDA Label]. |
| Marketing Status |
approved; investigational |
| ATC Code |
R06AX29; S01GX13 |
| DrugBank ID |
DB11591
|
| KEGG ID |
D09570
|
| MeSH ID |
C445659
|
| PubChem ID |
185460
|
| TTD Drug ID |
Not Available
|
| NDC Product Code |
42765-045 |
| UNII |
PA1123N395
|
| Synonyms |
bilastine |
|
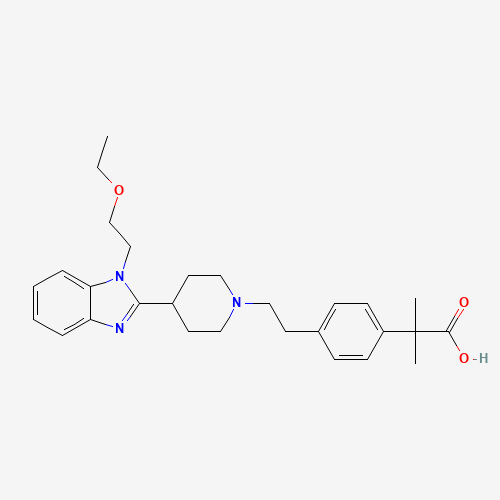
| Chemical Information |
| Molecular Formula |
C28H37N3O3 |
| CAS Registry Number |
202189-78-4 |
| SMILES |
CCOCCN1C2=CC=CC=C2N=C1C3CCN(CC3)CCC4=CC=C(C=C4)C(C)(C)C(=O)O |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
| ADR Term |
ADReCS ID |
ADR Frequency (FAERS)
|
ADR Severity Grade (FAERS)
|
ADR Severity Grade (CTCAE)
|
| Therapy partial responder | 08.06.01.064 | - | - | Not Available |
|
|
|

