| Pharmaceutical Information |
| Drug Name |
Defibrotide sodium |
| Drug ID |
BADD_D00598 |
| Description |
Defibrotide is the sodium salt of a mixture of single-stranded oligodeoxyribonucleotides derived from porcine mucosal DNA. It has been shown to have antithrombotic, anti-inflammatory and anti-ischemic properties (but without associated significant systemic anticoagulant effects). It is marketed under the brand names Dasovas (FM), Noravid, and Prociclide in a variety of countries. In the USA it is was approved in March, 2016 as Defitelio. |
| Indications and Usage |
Indicated for the treatment of severe hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), with renal or pulmonary dysfunction following hematopoietic stem-cell transplantation (HSCT).[label] |
| Marketing Status |
approved; investigational |
| ATC Code |
B01AX01 |
| DrugBank ID |
DB04932
|
| KEGG ID |
D07423
|
| MeSH ID |
C036901
|
| PubChem ID |
135565962
|
| TTD Drug ID |
D0FM2W
|
| NDC Product Code |
68727-800; 68225-107 |
| UNII |
L7CHH2B2J0
|
| Synonyms |
defibrotide | defibrotide (bovine) | defibrinotide | defibrotide sodium | defibrotide sodium (porcine mucosa) | JZP-381 | JZP381 | Defitelio |
|
| Chemical Information |
| Molecular Formula |
C20H21N4O6P |
| CAS Registry Number |
83712-60-1 |
| SMILES |
CC(C)C1=C(C=C(C(=C1)C2=NNC(=O)N2C3=CC4=C(C=C3)N(C=C4)C)O)OP(=O)(O)O |
| Chemical Structure |
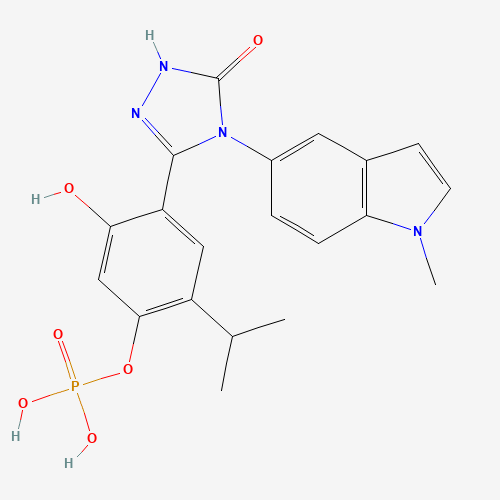
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

