| Pharmaceutical Information |
| Drug Name |
Ioxilan |
| Drug ID |
BADD_D01189 |
| Description |
Ioxilan is a tri-iodinated diagnostic contrast agent. Intravascular injection results in opacification of vessels in the path of flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs. |
| Indications and Usage |
When administered intra-arterially, Ioxilan is indicated for the following diagnostic tests: cerebral arteriography (300 mgI/mL), coronary arteriography and left ventriculography (350 mgI/mL), visceral angiography(350 mgI/mL), aortography(350 mgI/mL), and peripheral arteriography(350 mgI/mL).
When administered intravenously, Ioxilan is indicated for excretory urography and contrast enhanced computed tomographic (CECT) imaging of the head and body (300 and 350 mgI/mL). |
| Marketing Status |
approved |
| ATC Code |
V08AB12 |
| DrugBank ID |
DB09135
|
| KEGG ID |
D02161
|
| MeSH ID |
C055357
|
| PubChem ID |
3743
|
| TTD Drug ID |
D02BLO
|
| NDC Product Code |
Not Available |
| UNII |
A4YJ7J11TG
|
| Synonyms |
ioxilan | 5-(N-2,3-dihydroxypropylacetamido)-2,4,6-triiodo-N-(2-hydroxyethyl)-N'-(2,3-dihydroxypropyl)isophthalamide | Oxilan |
|
| Chemical Information |
| Molecular Formula |
C18H24I3N3O8 |
| CAS Registry Number |
107793-72-6 |
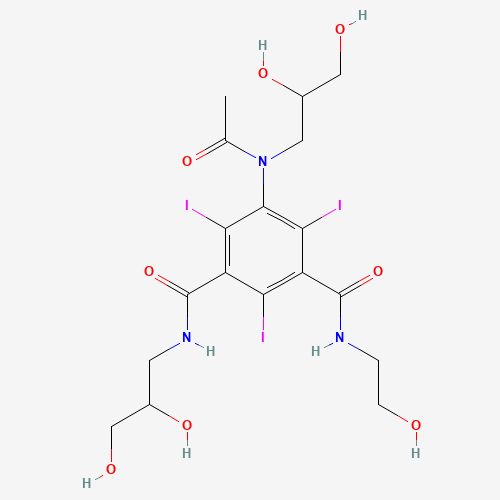
| SMILES |
CC(=O)N(CC(CO)O)C1=C(C(=C(C(=C1I)C(=O)NCC(CO)O)I)C(=O)NCCO)I |
| Chemical Structure |
|
|
| ADRs Induced by Drug |
|
|
*The priority for ADR severity classification is based on FAERS assessment, followed by the most severe level in CTCAE rating. If neither is available, it will be displayed as 'Not available'.
**The 'Not Available' level is hidden by default and can be restored by clicking on the legend twice..
|
|
|

